
News


Quintiles and IMS Health have successfully completed its merger to become Quintiles IMS Holdings, also known as QuintilesIMS.

The National Board of Medical Examiners (NBME) has introduced the Certification of Excellence in Clinical Research (CECR).

The discussion on the main characteristics and implementation of the new Clinical Trials Regulation is heating up in Europe.
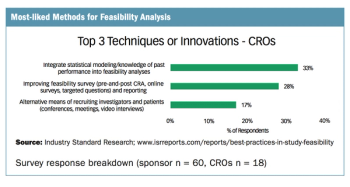
Survey drills down on the preferred methods of sponsors, CROs, and sites in conducting feasibility analysis.

Updated guidance incorporates the use of a patient-reported outcomes questionnaire-which could enable more endpoints and greater patient stratification for COPD trials.

Study collects first comprehensive metrics on current supply management and distribution practices.

An overview of clinical supply blinding methods in the context of the current research environment.
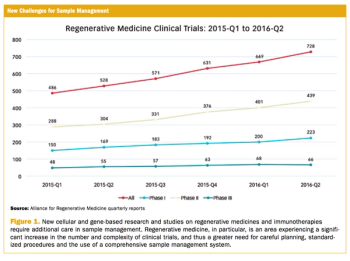
From basic blood draws to more involved samples, keeping accurate track and records is crucial for trials.
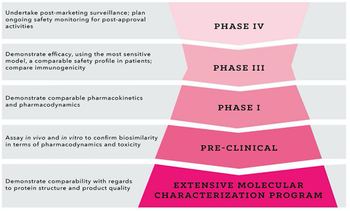
Using strategic planning to address hurdles in biosimilar development programs from the outset.
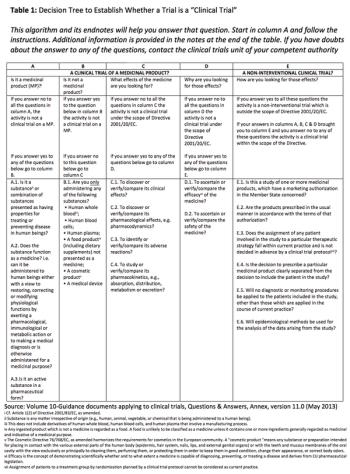
Examining interventional vs. non-interventional clinical study classification in the EU.

Click the title above to open the Applied Clinical Trials October/November 2016 issue in an interactive PDF format.


INC Research and the Center for Information and Study on Clinical Research Participation (CISCRP) have announced the winner of the 2016 “Inspiring Hope Ideathon”.

Industry professionals are searching for new technologies to support their trials following approval of an NIH policy supporting single IRB practices. Transparent and auditable systems can offer stakeholders the ability to more effectively monitor and report regulatory responsibilities.


Clinerion has released a new version of its Patient Recruitment System, designed to search electronic health databases of hospitals for eligible clinical trial candidates.

Gene-editing technology has the ability to give oncology researchers an effective treatment option in the fight against cancer. CRISPR-Cas9 is such a technology that is currently being used to study genes in cancer cells.
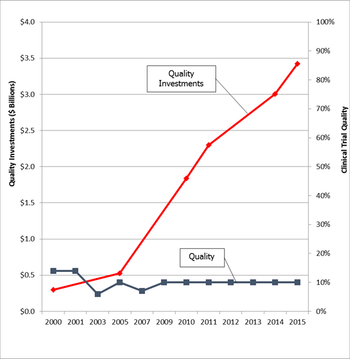
Most people in our poll said it was a service, but others disagreed. Find out more in this follow-up from Michael Howley PA-C, PhD and Associate Clinical Professor, Department of Marketing at the LeBow School of Business at Drexel University.

European Biopharmaceutical Enterprises (EBE) warns that Europe’s new drug ideas are going to waste due to a lack of innovative support in the sector. The EBE has offered several requests to stimulate investment, but attracting support from member state governments and the European Commission will be no easy task.
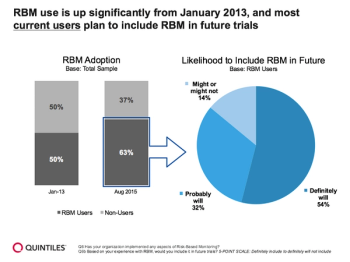
The use and awareness of risk-based monitoring is now impacting clinical trials of all sizes, indications and types as a core component more than ever before. Sponsors and sites are expected to expand its use to include all phases of clinical research and all therapeutics areas.

Functional dyspepsia is noted globally as being a common condition that currently has no specific therapy despite years of research. This condition is in need of an operational definition so that drug development and treatment trials can move forward with a more definitive trial design.

Acquisition of function service provider ExecuPharm addresses what PAREXEL sees as a growing trend among biopharm companies to use combinations of outsourcing models.



Cryoport has announced the integration of its Cryoportal logistics solution with Database Integration’s Integrated Cell Therapy Automated Network (iCAN) scheduling platform.


According to results of a new study by the Tufts Center for the Study of Drug Development, the development process for new diabetes and non-diabetes endocrine drugs is riskier compared to all drug development.

Technology innovations have been introduced into the clinical trials space that have the ability to change the trial experience for all involved. Egg’s TRIAL 360 platform is no exception, as its goal is to provide a networking platform to keep investigators and study stakeholders engaged in the trial.

Dr. Gonzalo Calvo has been re-elected by the Healthcare Professionals’ Working Party (HCPWP) of the European Medicines Agency (EMA) as its co-chair.