
The FDA Amendment Act is on its way toward implementation at years’ end. We reveal the facts on clinical trial disclosure for compliance with these new requirements.

The FDA Amendment Act is on its way toward implementation at years’ end. We reveal the facts on clinical trial disclosure for compliance with these new requirements.

With much happening in the clinical trials industry during 2016, Moe Alsumidaie looks back at some of the innovations that took place. He also looks forward to what the industry can bring us in 2017.

Over the last decade, many initiatives sponsored by various entities, including academic and clinical research centers have focused on efforts to streamline clinical trial eligibility and data collection. Oncology trial design is no exception as endpoints and eligibility criteria have also changed with the value of the data generated in early phase studies.


ERT has announced the acquisition of Exco InTouch.

A European research hospital has selected OmniComm’s TrialMaster electronic data capture (EDC) technology to integrate privacy-protected patient data from the hospital’s electronic health records.





The Dentistry School of the University of Istanbul, Turkey has joined Clinerion’s Patient Recruitment System platform.

Almac Clinical Technologies, part of the Almac Group, announced a partnership with inVentiv Health.

CRIO will participate in Medidata’s new eConnect Partner program for integration with its clinical cloud.


ERT announced an upgrade to its flagship platform for centralized respiratory clinical trials, MasterScope.
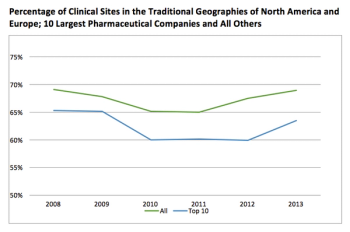
Most clinical trial activity for Phase III trials takes place in the traditional North American and European markets.
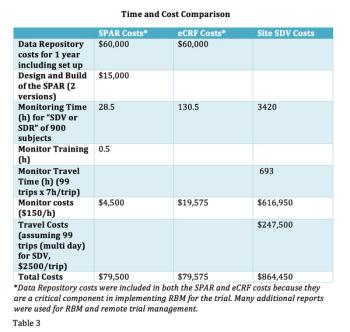
In a comparison study of source data review (SDV) methods, this new process demonstrates the value of using well-planned data visualization tools to provide better quality oversight versus remote eCRF review or onsite SDV.

A new SaaS platform for clinical trials, soon available to all pharma companies, aims to automate clinical supply, improve patient compliance and enable better medication adherence.


KMR Group has recently completed and released their 2016 Best Places for Clinical Research report series.

SCORR Marketing, in partnership with Applied Clinical Trials, recently conducted a survey to ascertain from industry professionals what big data comprises as well as its importance, how it is used today in the industry and how it may impact drug development services in the future.

The Council adopts revision encouraging sponsors to implement improved oversight and management of clinical trials.
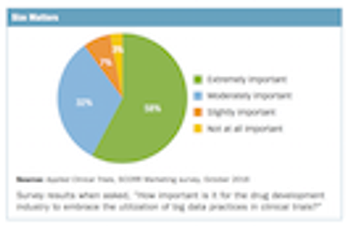
Survey collects thoughts from industry professionals on the current and future impact of big data in clinical trials.
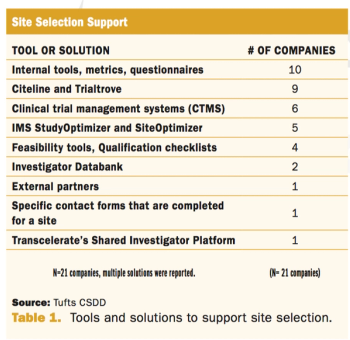
Assessing practices and inefficiencies with site selection, study start-up, and site activation.

How one large academic trial adopted a coordinating center model-helping drive early-model RBM gains

Q&A looks at the growing use of unstructured data in drug development and the evolving role of the clinical data manager amid the advent of real-world data as a research aid.

In its first published update since 2011, the CDISC Glossary Project Team has updated this resource, which includes hundreds of definitions for key terminology related to clinical research.

Click the title above to open the Applied Clinical Trials December 2016/January 2017 issue in an interactive PDF format.

The FDA’s Dr. John Whyte recently shared his perspectives on patient centricity at eyeforpharma’s Patient Centered Clinical Trials conference. He continues the discussion by exploring the definition of patient centricity and issues that not only the FDA, but also the industry is facing.
