
News


The European Medicines Agency (EMA) has released an update on its plans to revise the existing guideline on first-in-human clinical trials.

The Society for Clinical Research Sites (SCRS) has announced GlaxoSmithKline (GSK) as its newest Global Impact Partner (GIP).


Schulman IRB and iMedRIS Data Corporation announce a partnership to provide research organizations with integrated technology, consulting and IRB review services.

The American Heart Association (AHA) has announced the launch of a cloud-based Precision Medicine Platform in collaboration with Amazon Web Services (AWS).




PAREXEL has launched an Identification of Medicinal Products (IDMP)-focused solution, Liquent InSight, for biopharma companies.

Clinical Research IO (CRIO) is a website and Android app that allows users to conducts trial visits on a tablet or desktop, design source online, manage and schedule subjects and communicate with study monitors remotely.

A new group of collaborators has formed to determine the benefits and risk tradeoffs Parkinson’s disease patients are willing to make for a potential new therapy. Dr. Brett Hauber of RTI Health Solutions spoke to us about the collaborative.



The Leukemia & Lymphoma Society (LLS) has selected myClin for its Acute Myeloid Leukemia (AML) clinical trial.

CluePoints announced a partnership with the Metrics Champion Consortium, an association that develops standardized performance metrics to improve clinical trials.


INC Research announces its involvement in the Leukemia & Lymphoma Society’s (LLS) precision medicine Master Trial for the treatment of acute myeloid leukemia (AML).

In 2014, EU Regulation n.536/2014 represented a significant step toward transparency of clinical trials in Europe. However, despite the EMA’s policy 0070, which reinforced this concept, several aspects remain to be determined or evaluated, leaving room for additional requirements and local interpretation.
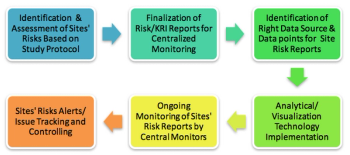
Centralized monitoring is a suitable way in which sites can identify and control investigational risks while improving performance. This new approach can not only help sponsors to monitor site performance, but also facilitate proper oversight resulting in good ROI.


eClinicalHealth Limited announced a partnership with Clinerion to offer a readiness certification to 27.5 million potential study candidates at hospitals in Turkey.

EUPATI celebrates its 5th Anniversary in Brussels by staging a free workshop.

The Drug Information Association (DIA) has released a video to introduce the two chairpersons of its 2017 EuroMeeting, to be held in Glasgow, U.K., from March 29-31

With pursuits in rare and orphan disease beginning to move more into the drug development mainstream, the opportunities and challenges in patient engagement and trial execution for these conditions are ever-evolving, as one strategy expert in the field discusses.



Medidata’s Clinical Cloud solution has been adopted by Japan’s Nobelpharma to power a clinical trial on sleep disorders in children with neurodevelopmental disabilities.

Schulman IRB will launch its new Central Oncology Review (COR) division this month.

FDA officials agree that large comparative clinical trials defeat the purpose of the abbreviated development program for biosimilars. Clinical testing is expected to gain market approval for most biosimilars, while emphasizing significant differences in developing biosimilars and innovator therapies.