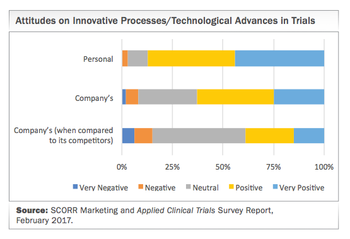
Highlighting the six broad technology themes poised to transform future R&D.

Click the title above to open the Applied Clinical Trials February/March 2017 issue in an interactive PDF format.


iCardiac Technologies has announced the launch of its Direct-to-Subject Pre-Coaching video tool.

The European Union effort to forge more cooperation among its national and regional HTA bodies is still a work in progress, as the process has produced varying views on the subject.

As part of its collaboration on type 2 diabetes with the University of Oxford in the U.K., Novo Nordisk is investing 1 billion Danish kroner over the next 10 years in a new research center.


PPD has announced the opening of a new clinical research unit for conducting Phase I and early development research in Las Vegas, Nevada.

With the new regulatory guidelines concerning GCP, questions have arisen as to how best RBM plans can comply. These questions and answers will assist sponsors in assuring compliance.


Aurinia Pharma has announced the selection of Worldwide Clinical Trials as its CRO for the AURORA study.


PAREXEL has announced the launch of its expanded Managed Access Programs service.

Examining the role of Clinical Research Malaysia in advancing industry-sponsored drug development in the country.


Clinerion has announced the release of its Patient Recruitment System Patient Finder tool for market access.

The 21st Century Cures Act, passed in December, contains provisions that many have questioned and criticized. This review of those provisions shows that progress in this sector is being made.


DrugDev has announced the release of the DrugDev Spark solution.

ArisGlobal has announced the release of the latest version of its clinical trial management software, agClinical 3.3.


A Brussels–based European Union conference on social rights set the stage for how access to healthcare should be widened with improved balance between innovation and affordability.

Nadir Benouali of MEDICODOSE Systems speaks on his experiences with investigational product nonadherence, its place in pharma and patient-centric ways to address it.

Lyft and Continuum Clinical announced a partnership that will provide transportation solutions for patients enrolled in clinical trials.

While wearable devices are mainly an advantageous way to collect data from monitoring a trial participant’s progress, there are scientific and operational considerations to be aware of.


Image Analysis Group (IAG) has announced a partnership with Boston Medical Center/Boston University School of Medicine (BMC/BUSM).

The Expert Determination standard of protecting patient privacy can answer the growing demand for better data for research and analytics in healthcare.

A revised version of the Common Rule will remove the need for additional informed consent when studying biospecimen data of clinical trial participants.