
An international panel of industry experts has concluded that randomized, controlled clinical trials provide the fastest and most reliable way to identify the risks and benefits of treatment candidates for infectious disease outbreaks.

An international panel of industry experts has concluded that randomized, controlled clinical trials provide the fastest and most reliable way to identify the risks and benefits of treatment candidates for infectious disease outbreaks.

MedNet Solutions announced that its eClinical solution, iMedNet, now leverages Amazon Web Services, as well as ClearDATA's security and infrastructure services.


Jeremy Sohn, VP, Global Head of Digital Business Development & Licensing at Novartis, delves into how biopharma is incorporating digital strategy into the execution of patient centric studies.



Accenture has announced a collaboration with BioCelerate, a subsidiary of TransCelerate, to develop a platform.

TrialScope, a company focused on clinical study transparency, has launched ATLAS Global Compliance.


PMG Research and Devana Solutions, LLC have announced a long-term subscription agreement.

The pharma industry agrees that the importance of entering data into an EDC as soon as possible following a subject is paramount. Slow site data entry can impact the credibility and usefulness of centralized monitoring data analytic reports.

Trial results demonstrated that stroke survivors were more likely to take an anti-blood clot treatment when using an artificial intelligence (AI) platform.
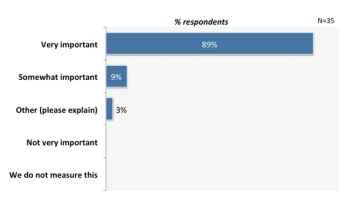
The pharma industry agrees that the importance of entering data into an EDC as soon as possible following a subject is paramount. Slow site data entry can impact the credibility and usefulness of centralized monitoring data analytic reports.



Chubb has expanded its global clinical trial liability insurance coverage for research organizations that face risks associated with managing clinical trials.

Clinical Research IO (CRIO) announced a collaboration with ArisGlobal

The European Medicines Agency (EMA) has developed a three-year plan to formalize, structure and develop interactions with the academic community.


Schulman IRB announced that research compliance and administration software provider iMedRIS Data is developing the technology to power Schulman’s forthcoming institutional biosafety committee (IBC) service.

A late-March volley of support from the European Union came in the form of a prize awarded to the new European Reference Networks, designed to connect patients with rare diseases to experts across Europe.


Medidata and pharma company Karyopharm Therapeutics announce an expanded partnership.

SCORR Marketing, in collaboration with Applied Clinical Trials, conducted a survey to ascertain the prevailing attitudes toward partnerships between sponsors and outsourcing companies in the health science industry. This report focuses on the type and extent of these collaborations.

The European Forum for Good Clinical Practice to examine the impact of current regulations and areas such as big data and quality management on so-called complex studies.

Application review will require closer cooperation between ethics committees and competent authorities in several areas.

Examining the evolution of protocol design and collaboration executing strategies.

The implementation of the International Organization for Standardization identification of medicinal products (IDMP) is a key step to ensuring that information on new innovations are identified and captured accurately within the regulatory framework and beyond.

Why following one consistent playbook for trial management should be at the core of outsourcing partnerships in drug development.