
News






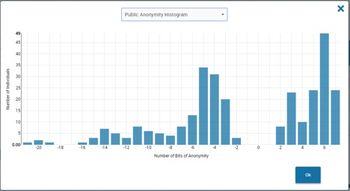
Examining the privacy of data and the mosaic effect in healthcare.




We discuss the pilot siteless models in clinical trial patient centricity with Gabriel Vargas, an executive at Amgen.




European experts in postmarking safety surveillance have the most to loose from impending EMA move post-Brexit.

Survey examines issues related to clinical trials quality and participation from the patients' view.

Q&A explores the implementation of the Trial Results Summaries Portal and the goals of the layperson summary program.

As Applied Clinical Trials celebrates its 25th birthday, longtime contributing author and columnist Ken Getz looks at the four truisms that still drive clinical trial operations today and into the future.

As our magazine embarks on the next 25 years of hopeful service covering the clinical trial enterprise, a quest for industry will be lessoning the complexity of drug development while remaining vigilant in protecting the patient.

Case study evaluates the use of a mobile device tool for simplifying clinical trial patient enrollment and data collection.

Audio study analyzes the communicative exchange factors between doctors and patients before enrollment-to better inform recruitment tactics.

As more strategic partnerships emerge, demands on CROs have intensified-resulting in the need for next-level competencies for project managers.

Examining the cost and patient recruitment and retention benefits of bringing clinical research procedures to the subject’s home.

As we mark a milestone year for our publication, several guest contributors look at the complexity issues in drug development still to overcome.

Click the title above to open the Applied Clinical Trials June 2017 issue in an interactive PDF format.


Chiltern has acquired Integrated Development Associates (IDA), a Japanese CRO specializing in integrating Japan and Asia into global drug development.


Certara announced a partnership with Beijing–based Phase I Unit, Peking Union Medical College (PUMC) Hospital’s Clinical Pharmacology Research Center.