
News







Peter O'Donnell discusses Brexit challenges and how the life sciences industry is affected.


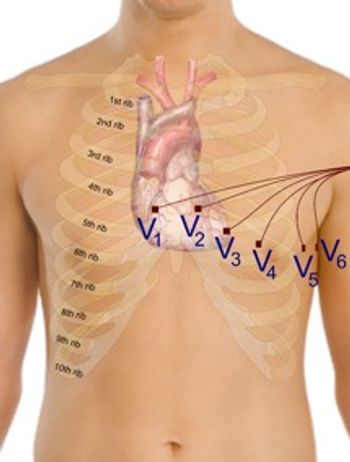
This article discusses the importance of good patient preparation, lead hook-up, and the quality checks that can be performed to ascertain that an ECG is of good quality.


Updated employee announcements, business news, awards and recognition in the industry today.

Outlining considerations on layperson summary writing of clinical trial results in Europe, and proposing a "reader-centered" approach to constructing these summaries.











Peter O’Donnell discusses the argument on whether or not European researchers should be allowed to continue using non-human primates in their research.

Click the title above to open the Applied Clinical Trials July/August 2017 issue in an interactive PDF format.



