
News



The use of Natural History studies in order to examine a rare disease that will help facilitate drug development programs.

FDA advisory committee made up of patients comes together to improve clinical trials and product development through better study design and greater participation.

Updated employee announcements, business news, awards, and recognition in the industry today.






Most physicians and many specialists have limited understanding of clinical trial data and research findings presented in prescription drug promotional materials for professional audiences.





Cancer vaccine-based immunotherapy may help overcome the resistance of certain tumors to immune checkpoint inhibitors, while immune checkpoint inhibitors may enhance the efficacy of cancer vaccine therapies.







Vienna joins growing list of bidders to land Europe's drug regulator; Amsterdam the early favorite?
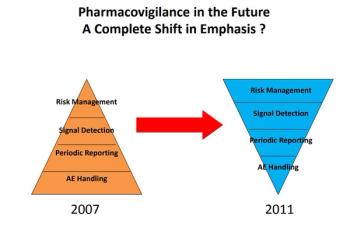
PV experts face new challenges as focus in field moves toward acting on real-world evidence insights.

Q&A explores the evolution of the patient-centric supply chain and how it's poised to impact the clinical trials landscape.
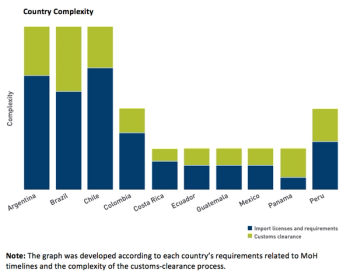
Supply chain strategies require a close look at the regulatory factors and the drug import hurdles and hopes in the region.
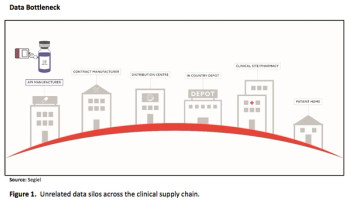
How a single-source temperature management strategy can support a drug’s quality and integrity in transit-a process as important as the destination.

Outlining a decision-making framework that integrates real-world signals with supply planning techniques to reduce risk and avoid potential interruptions.

Learn to partner effectively in the era of collaboration, where the benefits gained will outweigh the challenges if approached correctly