
News


Expert outlines approaches to preventing fraud in clinical research-a more prevalent problem that many believe.

Collaboration focuses on the use of a digital toolset in medication management and patient engagement during studies.

Dealmaking spurt and key upcoming regulatory guidances point the trajectory ahead in clinical research space.

Outlining the benefits of using blockchain technology across the supply chain to more securely record and distribute data to sites.

Weighing overall workplace trends in the life sciences with new survey findings and views on job satisfaction.







The decision of where to move the EMA was determined by a coin toss.












Updated employee announcements, business news, awards, and recognition in the industry today.



Machine learning technology is finding it’s way into today’s marketplace, specifically within business processes, by driving insurmountable value for corporations within the life science industry, writes Adam Ross Miller.
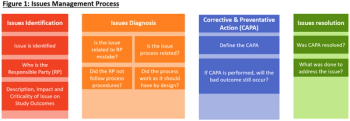
In this article, we will define a process on how to identify, diagnose, correct, and resolve issues in a QMS issues management repository.