
News


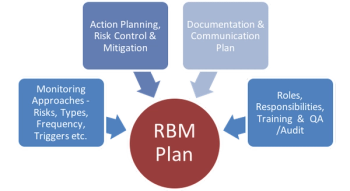
Sponsors and CROs are looking into implementation of a Risk Based Monitoring approach to their clinical trials to achieve the objectives related to enhanced data quality, better monitoring of patient safety, and creating efficiencies in overall operations.








Turning community clinics into research sites requires provision of personnel, processes, technology, and infrastructure to conduct clinical research.








Tensions mount across the European pharmaceutical scene over the fate of the EMA with just weeks before the final decision is going to be made on where it will be moved to.
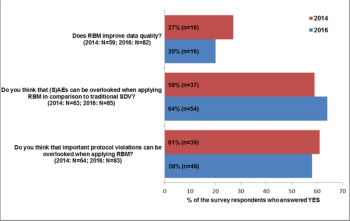
An RBM survey conducted in 2014 was repeated in 2016 in order to find out, how knowledge and practical experience with RBM have changed over that time period.


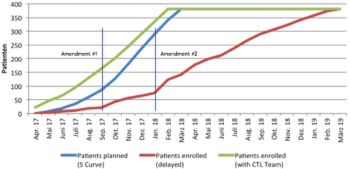
With several systems existing to measure performance, the most significant are the course of patient recruitment, how many patients complete the study, as well as the quality of the data generated.
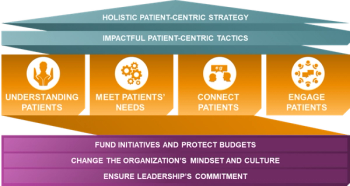
ZS Associates specify the four pillars of patient centricity that can help the demands and complexities of clinical trials.





