
News


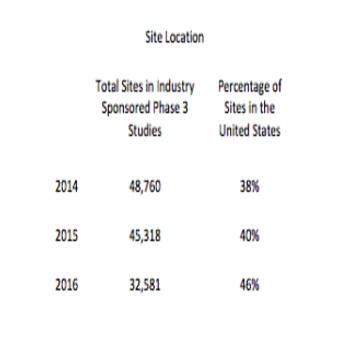
More recent ClinicalTrials.gov data indicate that the United States remains the central location for clinical trial activity.


An industry shift has led toward more insourcing and in-house resources to run trials.



Virtual care technology is emerging as a valuable tool for clinical trials, helping to improve recruiting and strengthen retention while optimizing resources for the overall study.



An expert view on how sponsors can formalize the use of real-world data and generation of real-world evidence to drive critical insights.
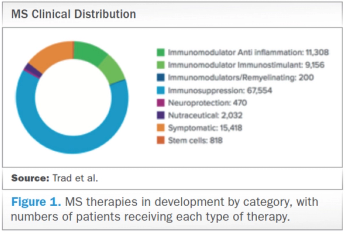
Examining the main challenges in designing and executing MS clinical trials and proposing mitigation strategies that may help alleviate these burdens.

To vie for success in the market for dermatologic therapies, companies developing biologics must navigate a series of significant challenges, including patient compliance and safety.

Findings from a new ACT and SCORR Marketing survey reveal a gap in the shift to true patient-engagement in clinical trials, but overall measures do signal growth in patient-centric activities.

Lisa Henderson reports on the J.P. Morgan Healthcare conference and the balance of investment and science.

The GS1 Standards will enable more efficient processes at institutions that are already using an electronic inventory system to manage investigational products.





The race to build the new EMA location in time for its move is becoming a crucial topic.

In this interview, Dr. Michelle Longmire, CEO of Medable, will discuss her perspective on the advancements of digital health in clinical trials.

As the EMA celebrates its 2017 highlights, the new year brings deeper challenges for the agency-including preserving the credibility of its patient-first mission.
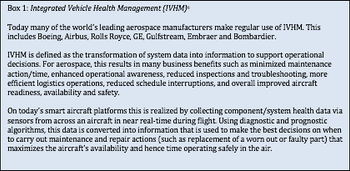
This article discusses real-time data capture and analytics in clinical trials.





A sex-bias that significantly impacts the advancement of women’s health exists in both preclinical and clinical research.
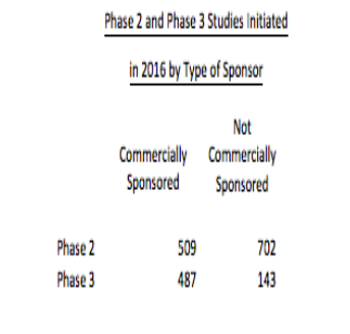
With the pharmaceutical industry looking for new investigators, a non-commercial clinical trial has become an under appreciated source.