
The topic of clinical trial quality and compliance continues to evolve in clinical operations, based on discussions at ExL’s 9th Proactive GCP Compliance Conference.

The topic of clinical trial quality and compliance continues to evolve in clinical operations, based on discussions at ExL’s 9th Proactive GCP Compliance Conference.
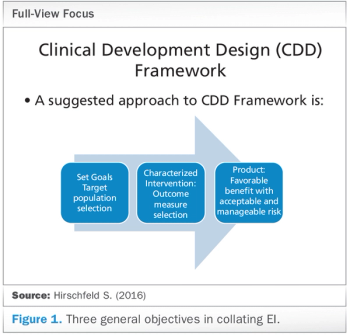
How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."

In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.

Jill Wechsler on the tug of war in accelerating orphan drug development.

Relocating the European Medicines Agency was always going to be hard-but no-one ever expected it to degenerate into farce.

The use of natural history (NH) studies early in clinical research can help facilitate development programs for orphan drugs.

Lisa Henderson discusses randomized clinical trials with active and non-active placebo treatments.

Click the title above to open the Applied Clinical Trials April 2018 issue in an interactive PDF format.

Though digital technology has improved the R&D process, when it comes to important clinical benefits and outcomes, modern biotech products have rarely shown the advantages of older generations of drugs.

Renewed attempts in the United States to win legislative approval for the right-to-try concept have again focused attention on that inevitably grey area between hoping a new medicine is going to be effective and demonstrating that it can be.




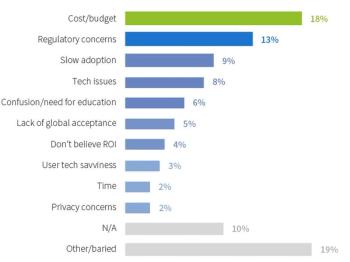
There are many benefits for sites adopting electronic informed consent and it is vital that site study teams are not bystanders in the move towards its use.



To encourage experimentation with more complex clinical trial strategies, FDA plans to launch a pilot program to assess complex studies proposed by sponsors.







Updated employee announcements, business news, awards, and recognition in the industry today.


Education Falsification has more consequences than not having a degree.

