
News



This article will analyze industry clinical trial initiatives and investigate strategies on how to improve impact on people.

The clinical trials industry craves blockchain technology to provide data safety and authenticity.

Biomarker and specialty lab data are increasingly being incorporated in FDA submissions given the robust insights they provide on key clinical objectives, including pharmacological effects, and drug safety and effectiveness.

Relocating the European Medicines Agency was always going to be hard-but no-one ever expected it to degenerate into farce.

With technology’s increasing ability to gather and analyze previously unmanageable data sets, and medicine’s forays into genomics and targeted therapies, the time of the master protocol may be at hand.


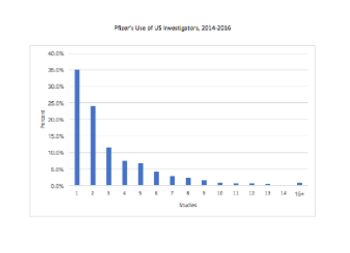
With Open Payments it is possible for Pfizer to better understand its own investigator usage pattern and how that pattern may differ from the practices of other pharmaceutical companies.

In this interview, Kevin Hudziak will expand on Lilly’s initiatives to change the face of clinical trials through patient engagement and education initiatives.



Digital media is becoming a crucial vehicle for finding “Patient X” for rare disease drug trials. Three areas in particular where digital media is helping with the detective work are explored.

Rare disease interests in Europe add more voices to push for greater backing of research incentives, infrastructure.

The ability to diagnose cancer at earlier stages of the disease is constrained by the specificity and mechanics of traditional diagnostic methods. The growing use of liquid biopsy technology may help overcome such barriers and potentially revolutionize cancer research, patient treatment, and survivor care.

Safeguarding trials from CRO turnover. How to ensure commitment of qualified resources through thick and thin.
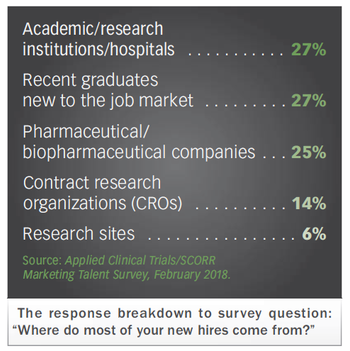
Exploring the latest hiring and retention practices in the life sciences, where roles are changing fast. Impact trends from new talent survey are revealed.

Click the title above to open the Applied Clinical Trials March 2018 issue in an interactive PDF format.







The rare disease community in Europe has come out fighting to defend its record-and its future-in the face of what it sees as growing threats to research.

By leveraging EHR data, the industry can transform how it conducts clinical research and delivers health care in the future.

