
Updated employee announcements, business news, awards, and recognition in the industry today.

Updated employee announcements, business news, awards, and recognition in the industry today.

On Europe Day, the agency itself is right now keener than ever to assess its worth and the value of its services to Europe and to Europeans.







In this interview, Munther Baara, Head of New Clinical Paradigms at Pfizer, will discuss his perspectives on how blockchain may impact clinical trials.
A discussion of the cost and time put into clinical trials.

There are several pharmacokinetic factors and peculiarities that should be considered carefully when designing and running first-in-human (FIH) studies for monoclonal antibodies.



Newest revelations suggest Risk Assessment Categorization Tools are now becoming therapeutic area-specific, as an oncology-based risk library is now surfacing.

BMS executive shares his perspectives on the current biomarker landscape, the role of translational medicine in accelerating this area of study, and how BMS is working to advance biomarker research across their R&D portfolio.

Peter O’Donnell explores potential parallels of the U.S. “right-to-try” debate in Europe.

Examining the unique standards and related challenges when assessing the safety and efficacy of cancer immunotherapy candidates.

Click the title above to open the Applied Clinical Trials May 2018 issue in an interactive PDF format.



An additional light has been shone on the "right-to-try" debate in Europe by a strongly-worded statement from Belgium's feisty health minister, Maggie De Block.

The British government continues to offer guarantees and reassurances about the post-Brexit future of its life-sciences sector despite the obvious failure so far to get even close to negotiating a withdrawal agreement with the EU.







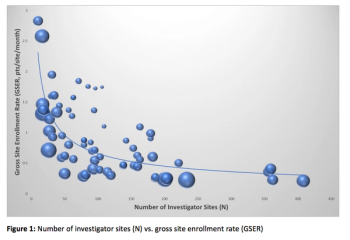
Adoption of the concept of Risk-Based Budgeting could help maintain the trial budget or even save the whole clinical trial.

Recent legislation authorizes further assessment of FDA eligibility policies and of NIH research standards.