
News






// You can adjust the form size here: var DrupalLeadsFormWidth = 870; var DrupalLeadsFormHeight = 2500;


Exploring that pivotal question for clinical investigators, sponsors, and global CROs.

FDA is testing various strategies to streamline research and regulatory oversight by looking to novel clinical trial designs to advance new treatments.

Although Biogen's Alzheimer's drug BAN2401 addresses buildup of amyloid plaques, investors and scientists look to reevaluate the plaque theory and search for a glimmer of hope in breakthrough treatments.
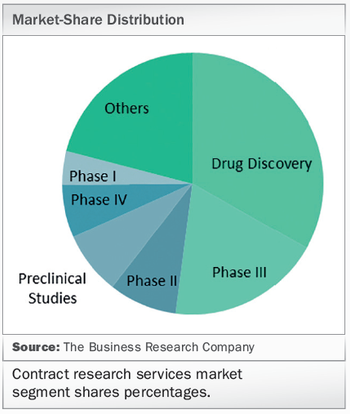
The Business Research Company publishes a report that the global market for clinical trial services to biopharmaceutical and medical device companies is forecast to grow at 12% year-on-year to 2021.

A continued struggle for healthcare and healthcare innovation to be taken seriously by the EU.

Switching from paper records to an electronic drug accountability IRT system can benefit sites during FDA trial site audits.

Assessing the benefits of using blockchain technology as a notary service in the network sharing of clinical data.
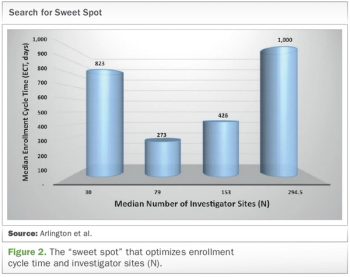
Analyzing data to reveal site performance patterns for better trial planning and execution.

Click the title above to open the Applied Clinical Trials July/August 2018 issue in an interactive PDF format.









The late-June announcement that Ireland is joining the Beneluxa Initiative on Pharmaceutical Policy might suggest renewed vigour for the drive to equip national governments with more clout in their pricing negotiations with international drug firms.



The general assumption that the Policy 0070 data releases were public data releases can be challenged since there is a mechanism to enforce the ToU, and this mechanism will continue to exist after the Agency moves to the Netherlands.

