
Feasibility, the evaluation of whether a particular trial can recruit enough of the right patients so that the study completes on time and within budget, is arguably the most critical part of the clinical trial process.

Feasibility, the evaluation of whether a particular trial can recruit enough of the right patients so that the study completes on time and within budget, is arguably the most critical part of the clinical trial process.







A look at current strategies from the industry that address the limitations in clinical trials.

The emergence of CTiD offers the potential to remove future clinical failures much earlier in the process-and pave way for gains in R&D productivity.

Click the title above to open the Applied Clinical Trials September 2018 issue in an interactive PDF format.

To support complex in-home clinical research and investigative sites, well-designed trials must produce the same high-quality data as traditional sites.

Industry experts look at how the specific needs of patients come into play when conducting gene-based screenings.

Alzheimer's Prevention Initiative's discusses a genetic counseling and disclosure model for its Generation Program.
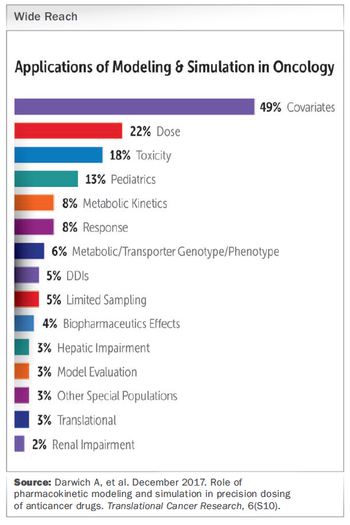
How the use of M&S in cancer trials from the outset can help address those critical “what if?” scenarios and accelerate oncology drug development.

The region is significantly underrepresented in clinical development activity targeting childhood cancer.

Insights on the challenges, activity, and motives in merging bridging research and clinical care.
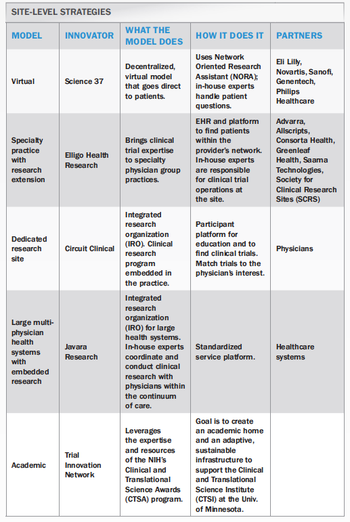
Site models that meet patient needs at the site level.

Partnership focuses on integrating research into the care continuum.

Case study highlights a new patient-centric enrollment model that uses a data-driven approach to identify qualified patients first.

Reexamining traditional cycle-time reduction strategies is critical for sponsors, CROs, and sites.





The relationship between European patients and drug developers has gone through many twists and turns over the years-and is still seeking equilibrium.

Comprehensive market landscaping includes disparate data sets from primary and secondary sources and is a time-consuming process that requires domain expertise and commitment.



