
Updated employee announcements, business news, awards, and recognition in the industry today.

Updated employee announcements, business news, awards, and recognition in the industry today.


Bracken Marketing has provided this infographic that categorizes more than 190 companies in this space by their focus.


There are several important factors which should be considered prior to resorting to international patient recruitment.


Healthcare professionals are engaged in an increasingly competitive fight to attract and retain clinical trial participants through the use of user experience (UX).

In this interview, Katie Mazuk, Global Head Investigator & Patient Engagement, will discuss her perspectives on improving patient connectivity.



Peter Benton, President and Chief Operating Officer of Worldwide Clinical Trials (Worldwide), will discuss areas of opportunity, CRO differentiation strategies, and organizational diversity.





At PanAgora’s Clinical Trials Patient Experience Summit, three main topics stood out; companies engrained patient centricity guiding principles in their operational models, the new concept of patient connectivity is emerging, and digital health is rapidly gaining ground in clinical trials.

A high-level working group of European regulators is trying to pull together a more coherent approach to real-world evidence-and is running into difficulties with the wide range of initiatives currently underway.

Although it is unlikely that mock demonstrators will be seen at any drug information or regulatory conferences, pharmacovigilance software is indeed having its “Salesforce” moment.

In this interview, Lisa Dilworth, as VP, Rare and Orphan Diseases at Synteract, will discuss common challenges with rare disease studies, how to encounter them, and how the rare disease space is evolving.




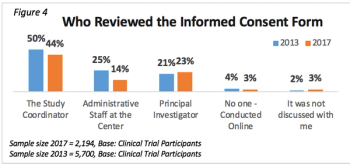
To inform our understanding of public and patient trust and confidence, this article provides a high-level summary of the results of two studies monitoring trends in international public and patient attitudes and perceptions.

FDA initiatives aim to increase biomarkers and early advice to sponsors for more efficient and cost-effective clinical trials for developing targeted therapies, including orphan therapies.

Pharma and diagnostics companies need to strengthen alignment in Alzheimer’s disease research-to help turn science and data into actionable medical innovations.
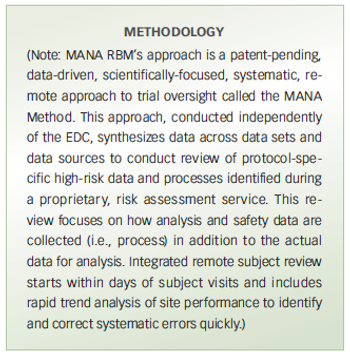
Pilot study compares a risk-based monitoring and remote trial management method with traditional on-site source data verification for trial oversight.

All of Europe, from MEPs to WHO, aims to develop a comprehensive health policy, with a current priority on nutrition and physical activity.

Blockchain is moving toward definitive uses in clinical trials to enhance clinical supply capabilities, with the potential of enabling data ownership for patients.

Click the title above to open the Applied Clinical Trials November 2018 issue in an interactive PDF format.