
News
Advertisement


Advertisement

October has offered a striking spectacle of contrasts in Europe's ponderous attempts to construct a comprehensive policy on health.


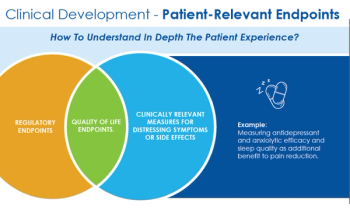
This article delves into the why, when, and how a patient-centric approach should be adopted for both the benefit of patients and the successful development of novel and innovative medicines.
Advertisement










What technologies ease clinical trial laboratory sample and data management, and what are the benefits?





Dr. Tshaka Cunningham, Associate Director of Scientific Collaboration for DIA, will discuss his perspectives on advancements in gene editing therapies and their impact on the industry.






Updated employee announcements, business news, awards, and recognition in the industry today.


Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
4
Accelerate Clinical Trials with AI-Enhanced Financial Management
5