FDA prioritizes to maintain, and continue to improve, the efficient and effective drug development and review system the agency has established in recent years.

FDA prioritizes to maintain, and continue to improve, the efficient and effective drug development and review system the agency has established in recent years.
An increase in use of EHR systems at clinical investigative sites adds more expectations for clinical trial sponsors to comply by regulatory requirements.

Case study demonstrates how one global mid-sized sponsor is testing the RBM waters.

Using data analytics to introduce parallel processes to optimize and accelerate clinical trials.
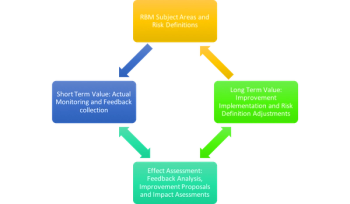
RBM is an emerging area which gives endless opportunities to explore, transform, and evolve.



DIA Europe proves to have excellent timing to learn more about the latest developments in drug development, the current status of Brexit, the plans of the National regulators and EMA, on-going discussions on pan-European HTA, and the trends for 2019.

2018 has been an exciting year in the clinical trials industry, as we have seen many changes in novel concepts, and the evolution of some concepts to the point where initiatives and pilots are crystalizing into common practice.
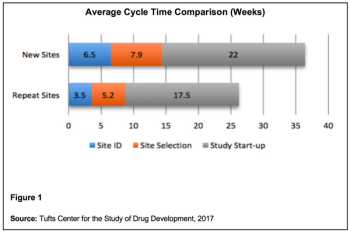
Using data analytics to introduce parallel processes to optimize and accelerate clinical trials.



Updated employee announcements, business news, awards, and recognition in the industry today.

In this Q&A, Helen Matthews, Jessica Morris, and Bruce Hellman offer their perspectives on the priorities, opportunities, and challenges of patient centricity in real-world evidence collection.
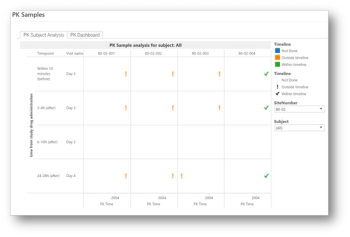
Risk-based approaches to monitoring (RBM) have become widely accepted in the clinical trials industry.

As the risks grow of a disorderly UK withdrawal from the European Union, the warnings about what could go wrong for the supply of medicines in Europe become ever louder.



Remote trials have the potential to increase recruitment, reduce attrition, and make patients feel more engaged with the research.

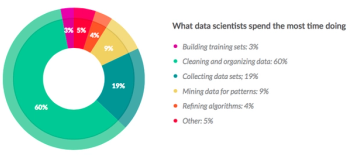
The world of data management today is practically synonymous with electronic data capture (EDC).



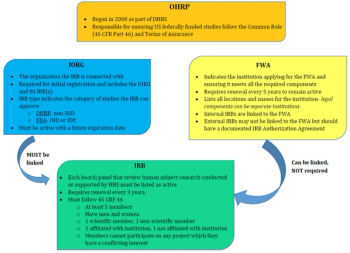
This article will focus on specific requirements to ensure the Protection of Human Research Subjects (Title 45 CFR 46).
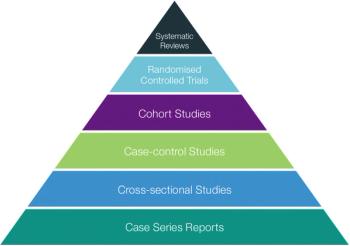
Real-world evidence (RWE) is needed in addition to clinical trial data to understand drug effectiveness in a real-life setting and to profile patient populations in terms of their clinical characteristics and drug utilization.

In the following Q&A, Guillaume Marquis-Gravel, MD, and Kevin Monroe discuss the advancements and related challenges of incorporating new technological tools in drug development, including eClinical approaches currently undertaken in the foundation’s own studies.