
News












How much does the EU really care about health, and about the infrastructure that is a precondition to successful healthcare and healthcare innovation?








The right-to-try gale is now blowing at full strength in the US and is likely to prove a straw in the wind that could become a haystack.
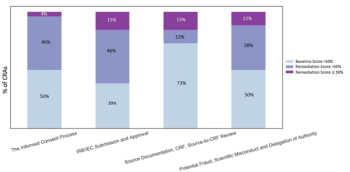
In this article, we will demonstrate a methodology that resulted in improving CRA performance.


Updated employee announcements, business news, awards, and recognition in the industry today.


Certified principal investigator certification as a predictor of major and critical protocol deviations in clinical trials.

Liquid biopsy, typically non-invasive blood-based tests for circulating tumor DNA and circulating tumor cells, has been long-hyped as a potential game-changer for cancer treatment.


