
News







The adoption of automated payment methods for phase I trials will allow for more effective clinical trials.






Updated employee announcements, business news, awards, and recognition in the industry today.

Agile Product Development Methodology holds great promise for use in Adaptive Clinical Study Development.


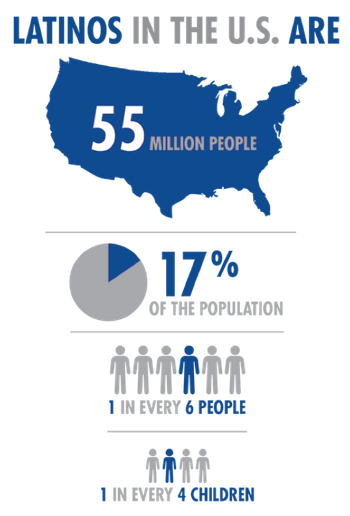
Despite the evidence that the Hispanic population is increasing at a formidable rate, they not only face disadvantages in health care access, but are significantly underrepresented as participants in biomedical research.

These three steps should take place in order to ensure an optimal site selection.




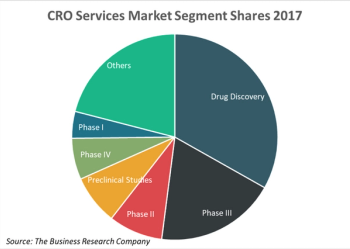





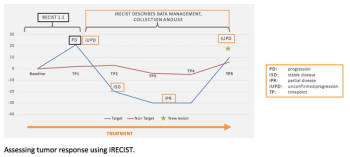
Cancer immunotherapies require different standards for evaluating their safety and effectiveness. Understanding these standards-and the other major challenges of immuno-oncology studies-is critical to drug development success.