
News








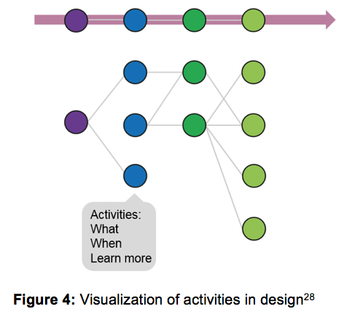
How the Clinical Development Design (CDD) Framework can offer repeatable, reusable clinical designs based on "enabling information."









At the October Metrics in Patient Centered Drug Development conference hosted by DIA, many of these key constituents gathered to talk about how to implement and measure the impact of patient input.

The operational subject of how the centralized authorization procedure is going to work when the UK is no longer part of the European Union is being discussed.

Steve Young looks at the tremendous benefits that organizations stand to reap by effectively implementing the core principles included in the ICH update and the significant opportunities that the application of intelligent analytics and centralized statistical monitoring may present.
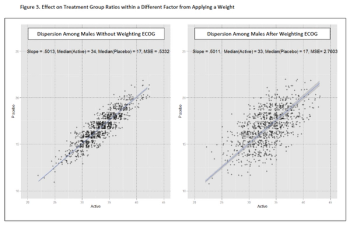
An overview of quantifiable techniques when choosing the parameters in dynamic randomization for clinical trial enrollment.






Click the title above to open the Applied Clinical Trials November/December 2017 issue in an interactive PDF format.

