
News







The release of the ICH E6 R2 guidance, which outlines a new approach to clinical trials operations, will have a major impact on the industry, writes Patrick Hughes.

Executives from Edgerton Data Consulting and MedSurgPI discuss biopharmaceutical and medical device companies outsourcing one or more aspects of their clinical trials.



In this interview, we speak to Dr. Joanne Waldstreicher, Chief Medical Officer of J&J’s Office of the Chief Medical Officer (OCMO), to discuss its data sharing program via the Yale University Open Data Access (YODA) Project.





Executives from Atlantic Research Group discuss the importance of CRAs in rare disease clinical trials.



How can manufacturers ensure their clinical trial methods are evolving along with their product portfolio? It starts by taking a patient-focused approach to trial design, writes Susan Weidner.

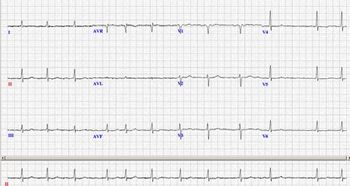
This article discusses atrial fibrillation (Afib) detection and ECG recordings of the arrhythmia.

SCORR Marketing conducted a survey in partnership with Applied Clinical Trials to measure attitudes and beliefs of those in the health sciences as they pertain to the globalization of clinical trials. Specifically, this report seeks to identify the challenges faced in operating multiregion clinical trials and the difficulties inherent in setting standards in emerging markets

Pressure is growing on clinical trial organizers to involve more pregnant women in drug research.

Q&A explores the importance of new vehicles to identify and recruit physicians seeking non-interventional research opportunities.
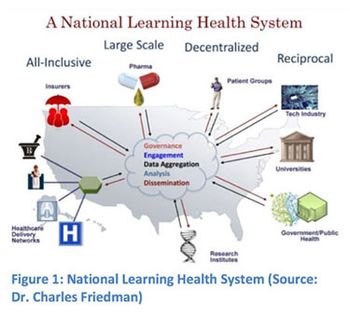
Outlining the ways FDA is adopting electronic practices and initiating efforts to simplify the submission process for new drug and generic drug applications.

Traditional and new players are seeing growth and opportunity as the integration of clinical research and healthcare intensifies.

As industry headlines buzz with drug pricing uncertainty and M&A speculation, sponsors can boost productivity on the R&D front through more conscious management of outsourcing spend.
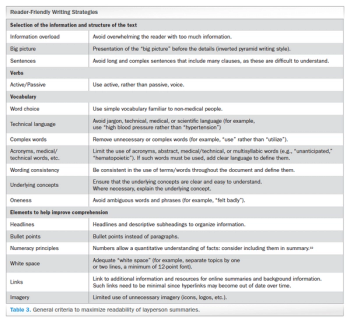
Analyzing the debate around writing and constructing layperson summaries of clinical trial results-and proposing a “reader-centered” approach.
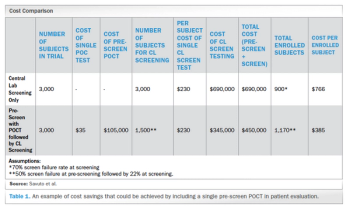
Retrospective analysis of clinical trial enrollment data evaluates the effectiveness of point-of-care testing in reducing later-screen failures.

New survey data, coupled with recent research, spotlight the current views and concerns around multi-regional studies.