
News


Translational Drug Development (TD2) and Deep 6 AI have agreed to collaborate in the fields of oncology and fibrosis.


In order to successfully support the globalization of clinical research, sponsors and CROs must empower global research sites and treat them as valued business partners.

Interest in improved project management practices is growing in the fast-moving pharma industry.


SCRS announced that Medpace has extended its commitment as a Global Impact Partner by two years.



Takeda’s Atul Mahableshwarkar offers insight into central nervous system drug development and how biomarkers can improve therapy development of this discipline.

TrialScope has released its second Clinical Trial Disclosure Maturity Survey for sponsors to perform self-assessments of their disclosure practices.

The European generic drug industry homed in on the opportunity to “help the EU and member states develop effective policies that support access to medicines for patients.”



endpoint Clinical and Clinical Ink have teamed up to integrate IRT and eSource to create a seamless user experience for investigator sites.

CROS NT announced an extension of its partnership with Medidata to coincide with the launch of its RaveX team.


Orbita, a provider of voice-first software for connected home healthcare, announced a collaboration with ERT to research new approaches to data procurement and management.


ERT has introduced a respiratory clinical trial solution to capture, measure and analyze Lung Clearance Index data.

Approaches such as machine learning, AI and neural networks are still in their infancy, but with AI companies rewriting the code for drug discovery, the implications in pharma are likely to be far ranging in the coming years.


Italy-based CRO Exom Group has announced the launch of its electronic informed consent solution, Genius Engage.

With the availability of workflow-based study start-up tools, proactive planning is within reach for stakeholders who view this function as pivotal to improving clinical trial quality.
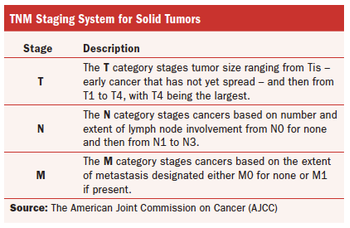
Examining the potential of such diagnostic technologies in reshaping oncology trials.

Updated employee announcements, business news, awards and recognition in the industry today.

Functional service provisions can provide sponsors and CROs with a way to efficiently manage clinical trial outsourcing methods.
Ensuring the supply of clinical drugs during a time of crisis is a necessary moral and ethical action to take. This decision-making framework integrates real-world signals with supply planning techniques to reduce supply chain risk and avoid potential supply interruptions.

Cancer's R&D Convergence will highlight the changing landscape for clinical trials in this therapeutic area based on the promise of the Cancer MoonShot initiative, along with new oncology immunotherapies.