
California-based physician group, Desert Oasis Healthcare has announced the establishment of a Center for Research and Healthcare Innovation in collaboration with Altura.

California-based physician group, Desert Oasis Healthcare has announced the establishment of a Center for Research and Healthcare Innovation in collaboration with Altura.


San Francisco, CA – endpoint Clinical, a leading global interactive response technology (IRT) company, has announced a significant enhancement to its proprietary PULSE platform, reshaping the way IRT is applied to clinical trials.

The global skills shortage in the clinical research sector is being exacerbated in the U.K. by the uncertainty created by Brexit.

Synexus and Radiant Research are merging their operations to form a single global site network of 185 sites with 1,500 employees.

Western IRB announced an alliance with global professional services firm Huron.

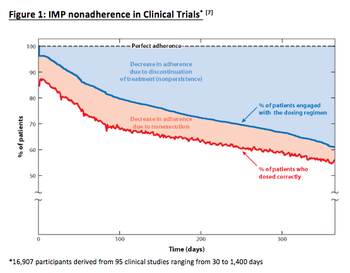
As clinical trials remain costly and continue to increase, the promise of novel initiatives gives hope that trial duration and cost impact will drop. However, biopharma continues to overlook one element that affects study timelines: patient non-adherence.

Cliantha Research has announced the acquisition of Inflamax Research. Inflamax aims to use its inflammatory disease research and natural allergen Environmental Exposure Chambers (EEC) to assist Cliantha in conducting asthma, allergy, ophthalmology and tobacco research plus specialized pulmonary/respiratory therapeutic studies.Read the full release here


Veeva Systems has announced the release of Veeva Vault Exchange, TransCelerate has selected this platform.

Novotech announced that it has signed a Memorandum of Understanding (MOU) with South Korean medical institution, The Asan Medical Center.

Cliantha Research is proud to announce the acquisition of Inflamax Research. Inflamax has locations in Toronto, ON; Neptune, NJ and Newark, NJ. Cliantha Research will have the capabilities to perform Phase I, II, III and IV clinical research in North America. Inflamax’s world renowned expertise in inflammatory disease research and patented natural allergen Environmental Exposure Chambers (EEC) shall allow Cliantha to conduct Asthma, Allergy, Ophthalmology, Tobacco research plus specialized pulmonary/respiratory therapeutic studies.


Initial findings from a DIA study of patient-centric initiatives in drug development reveal pharma’s desire to move toward true patient-centricity, but approaches to implementation are varied.

Certara announced the launch of a new solution for preparing, analyzing and submitting pharmacokinectic data in Clinical Data Exchange Standards Consortium (CDISC) format.Read the full release here

A certified CDISC provider and leader in pharmacometrics, Certara has developed a new solution which will enable sponsors to achieve new compliance requirements effortlessly


ePatientFinder and Rep Network have announced a partnership that aims to provide clinical trial access to patients nationally.


BioPlan has released its updated textbook, A Quick Guide to Clinical Trials, 2nd Edition.

Updated employee announcements, business news, awards and recognition in the industry today.


Medidata announces the acquisition of eClinical technology company, Mytrus Inc. Medidata will add Mytrus’ eConsent solution, Enroll, into the Medidata Clinical Cloud.

Why taking a right-sized approach to real-world studies will help keep sites and patients around for the duration.
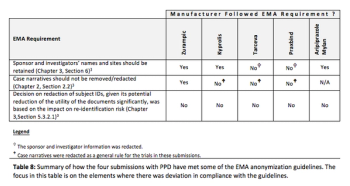
Examining early learnings from approaches used to comply with EMA’s requirement to publish anonymized versions of clinical study reports and other submission documents, including how privacy protection was balanced against data utility.

In today’s value-driven healthcare system, real-world studies are increasingly becoming mandated by regulators as a condition of approval for new medicines.

In continuing our series on patient centricity, we are adding the digital perspective from inside of a biopharmaceutical enterprise. In this interview with Jeremy Sohn, VP, Global Head of Digital Business Development & Licensing at Novartis, we will delve into how biopharmaceutical enterprises are incorporating digital strategy into patient centric study execution, and elaborate on some of the cultural challenges of adopting novel study methods.


YPrime has announced the release of its of its data-driven electronic clinical outcome assessment (eCOA) technology platform.