
How to assure that software used in clinical trials will support regulatory scrutiny during pre-approval inspections and application review.

How to assure that software used in clinical trials will support regulatory scrutiny during pre-approval inspections and application review.

Examining the role of Clinical Research Malaysia in advancing industry-sponsored drug development in the country.

Outlining current regulatory issues and views surrounding expanded access to investigational products in the U.S.

How CROs and sponsors can step up their relationship game.

Click the title above to open the Applied Clinical Trials April/May 2017 issue in an interactive PDF format.


PAREXEL announced the launch of its patient sensor solution for capturing, transmitting, storing and visualizing study subject data in clinical trials.
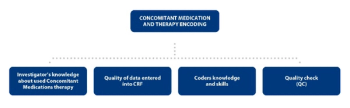
In Case Report Forms data regarding Concomitant Medication, therapies can be presented in multiple ways. Medical coding is a common task that can ensure the consistency of the captured data.

The last piece in our three-part series on improving efficiencies and sponsor/CRO collaboration through advanced CTMS, reveals a way in which CROs can best their competition in the marketplace.

Stakeholders of Project Data Sphere authored a Sounding Board article in the New England Journal of Medicine urging the responsible sharing of cancer trial data with global researchers.

Medidata announced that The Leukemia & Lymphoma Society has selected Medidata’s Clinical Cloud platform to power its trial for acute myeloid leukemia (AML).


PPD announced the launch of a clinical research associate apprenticeship program.


We previously covered the challenges that biopharmaceutical enterprises are facing when it comes to developing CNS medical products at ExL’s 2nd CNS Clinical Trial Forum. In this interview, Glenn Morrison, Executive Director of Clinical Development at Zogenix, will discuss how biopharmaceutical enterprises can apply techniques used in oncology clinical trials in CNS development.

Oracle Health Sciences released its Data Management Workbench Cloud Service that aims to streamline end-to-end clinical data flows across R&D.


The Association for the Accreditation of Human Research Protection Programs has announced the accreditation of nine research organizations in the U.S., Belgium, China and Taiwan.


WIRB has launched a new tool designed to assist participating institutions in complying with NIH’s new single review policy for multi-site clinical trials.


The second in our three-part series, Improving efficiencies and sponsor/CRO collaboration through advanced CTMS, explores how technology can foster better collaboration.

Cloud-based clinical analytics and software solutions provider, Algorics, has announced the newest release of its risk-based monitoring platform Acuity.

Women are often underrepresented in HIV clinical trials, but they appear to prefer finding out about trials by means of peer-to-peer communication, according to new analysis.



CRF Health has released the results of a new ‘State of eConsent’ survey.

OmniComm Systems and Netherlands-based development company, the Centre for Human Drug Research (CHDR), have announced a collaboration.
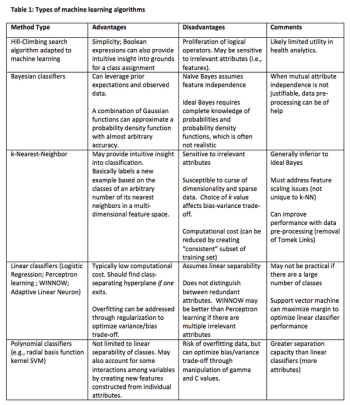
With the inherent limitations of traditional data sources, this overview explores the definitions and strategic fundamentals that inform machine learning and modeling techniques.