
Wingspan Technology has announced a partnership with Tokyo, Japan-based system integrator Information Services International-Dentsu (ISID).

Wingspan Technology has announced a partnership with Tokyo, Japan-based system integrator Information Services International-Dentsu (ISID).


PSI CRO has chosen goBalto’s end-to-end study startup platform for conducting clinical trials from site identification, feasibility and selection through to site activation.

Updated employee announcements, business news and recognition in the industry today.

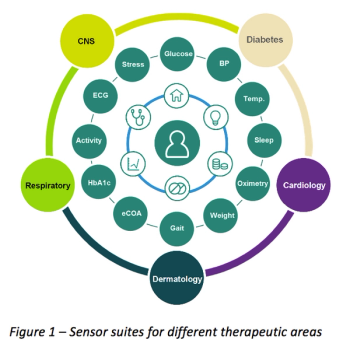
Investment in mHealth and the adoption of wearables are significant initiatives that are changing the clinical trial process toward a more patient centered approach. These wearable technology initiatives have the potential to be the most innovative advances in drug development.

Takeda Pharmaceuticals has entered into a partnership agreement with PRA Health Sciences.


The Society for Clinical Research Sites (SCRS) and the National Association of Veterans’ Research and Education Foundations (NAVREF) have announced a partnership.


Thermo Fisher Scientific has announced the opening of its new GMP standard facility in South Korea.



MediData announced that Techfields Pharma has selected its Clinical Cloud solution to power a Phase II schemic stroke study.

Veeva Systems has unveiled the results of its 2016 Paperless TMF Survey: CRO Report.

The Society for Clinical Research Sites (SCRS) has announced Bioclinica as its newest Global Impact Partner.

One year ago, the China Food and Drug Administration (CDFA) was pushed to accelerate its activities to promote new or updated regulations and guidelines. Since then the results have been smoother processes for innovative drug development in China.


iCardiac Technologies and SNBL Clinical Pharmacology Center have announced a collaboration on cardiac safety studies in Japan.


The Society for Clinical Research Sites (SCRS) has chosen Acurian as their corporate sponsor for its SCRS Ambassador Program in Poland.
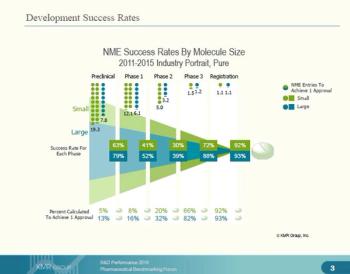

The Pharmaceutical Benchmarking Forum has released data concerning R&D success rates of large and small molecules.


ERT has updated its Insights Cloud trial oversight solution to enable sponsors and CROs to collaborate by sharing electronic monitoring visit reports and issue management, tracking, and reporting.

Patients of gastroesophageal reflux disease (GERD) that have been frequently diagnosed, or misdiagnosed, have been dissatisfied with the available treatment. To resolve this issue, industry players must define meaningful endpoints for future trials, incorporate patient reported outcomes, and apply biomarkers.

TransCelerates work on their clinical trial Quality Management Systems (QMS) initiative began about one year ago. This article summarizes findings from a recent DIA publication about the initiative and evaluates potential issues during QMS framework implementation.


Medidata and SHYFT Analytics have partnered with a goal of increasing access to healthcare data analytics in clinical trials.

Following the UK’s decision to leave the European Union, the result has been uncertainty and speculation as pharma attempts to understand its implications. How this change will effect the pharma and life sciences industry as well as the European community remains to be seen.