
News


The Clinical Trials Transformation Initiative (CTTI) have released recommendations for hospital acquired and ventilator-associated bacterial pneumonia (HABP/VABP) as part of its antibacterial drug development program.


CRF Health has acquired Entra Health, an international digital health and IT solutions provider.


The KMR Group has completed its industry analysis on the comprehensive cost of clinical trials.

The uncertainty surrounding where the agency and its 890-member staff will call home next may linger for months, if not years.

Janssen releases key findings from its pilot study evaluating the use of electronic informed consent technology in clinical trials.

More precision models in connecting patients to clinical trials and treatment options are vying to close the ever-elusive recruitment gap.

Survey delves deep into what constitutes true game-changing innovation in clinical development.

A proposed operational framework to support digital- and mHealth-based clinical trials in Africa.

Exploring the benefits from cross-company data sharing for study planning and investigator selection.
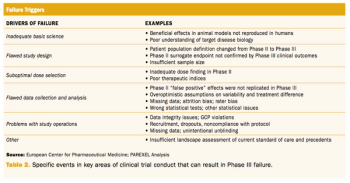
An examination of recent failures in Phase III studies and innovative approaches to reduce risk.

Research reveals a need for sponsors to be much more consistent, disciplined and focused in their CRO usage.

Study measures the differing judgment levels between clinical investigators and drug safety experts.








Click the title above to open the Applied Clinical Trials August/September 2016 issue in an interactive PDF format.

Start-up biotech selects Medidata Rave for Phase I and II trials of its investigational compound to address solid tumors and blood cancers.



INC Research announces the launch of its Vaccine Catalyst Site Network.


INC Research has achieved the “Bronze Standard” from the IAOCR’s Clinical Operations Workforce Quality Accreditation program.
