
The challenges of patient recruiting must be addressed if medical breakthroughs are to be made. Education, simplicity and patient feedback could go a long way in improving the recruitment process.

The challenges of patient recruiting must be addressed if medical breakthroughs are to be made. Education, simplicity and patient feedback could go a long way in improving the recruitment process.

With North America and Europe accounting for the majority of active clinical trial sites in 2016, the need for globalization is increasing. Brazil, Russia, India and China are emerging markets with potential, but not without challenges.


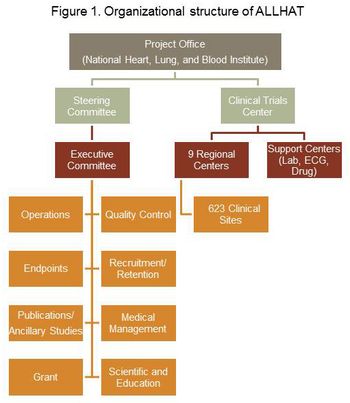
The largest antihypertensive trial ever conducted throughout North America, ALLHAT, was organized using a coordinating center model for oversight. Regional coordinators played a key role in carrying this trial out.

eClinicalHealth and Clinerion have collaborated to offer their two clinical trial platforms in tandem to hospitals and sponsor companies/CROS.


UPS announces an expansion of its shipping capabilities to support clinical trials.

Respiratory disease trials are difficult to initiate, both in terms of patient recruitment and accurately collecting data. Alternative methods are required to improve outcomes and develop new treatments for respiratory diseases.

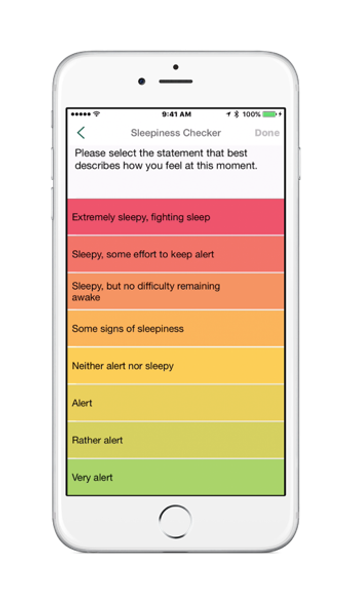
Adam Amdur of the Sleep Apnea Association speaks with Moe Alsumidaie about his company’s SleepHealth mobile app study and the impact of mHealth in clinical trials.

To ensure success of clinical trials, biopharmaceutical companies must explore leveraging a variety of channels to prepare investigators. Such a process has the potential to save money and provide effective training for future trials.



Quintiles opened its global Solution Design Studio, located in Research Triangle Park, North Carolina, where expert teams will collaborate to create technology solutions.

eClinicalHealth announced the the final results of the VERKKO remote online Phase IV clinical trial for diabetes.
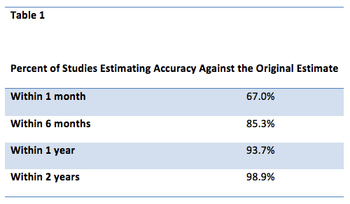
With a unique business model, pharmaceutical companies and their subcontractors need to find every way possible to shorten drug development times consistent with patient safety. The challenges facing commercially oriented organizations developing prescription drugs are substantial.




The Association for the Accreditation of Human Research Protection Programs today announced that it has accredited four additional research organizations.

eClinicalHealth Limited, with support from ICON eCOA practice, has released a methodology to validate and standardize patient questionnaires across different data capture tools.

ClinDatrix implements Oracle Health Sciences InForm 6.1 and Oracle Argus 8.0, electronic data capture technology.



Chiltern has adopted ePharmaOne, a proprietary site management platform offered by e-clinical solutions provider ePharmaSolutions.


ERT announced the release of its Insights Cloud trial oversight solution for clinical trial sponsors and Clinical Research Organization (CROs).
