
European forum tackles the problem of limited elderly patients involved in clinical trials.

European forum tackles the problem of limited elderly patients involved in clinical trials.
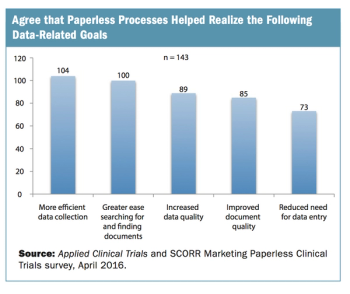
Survey reveals mixed adoption of paperless data collection in clinical trials, pointing to the need for greater alignment of new eClinical technologies and study conduct.

Recent FDA draft guidance pushes for the use of electronic health record data in clinical investigations-and synching EHRs with research systems.

CDER official comments on the significance of ICH's new alternative path for identifying the cardiac safety issue of QT prolongation in non-cardiac drugs.
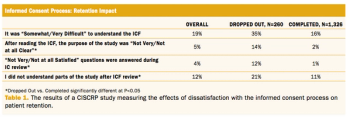
Why electronic informed consent is key to supporting today’s patient-centric mantra in clinical trials.
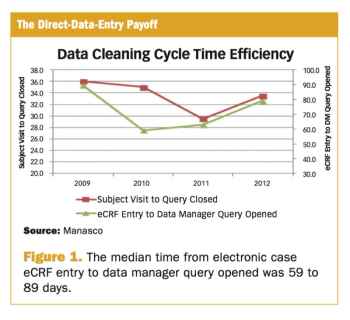
Outlining those technologies best able to raise the data and process quality of risk-based monitoring.

Click the title above to open the Applied Clinical Trials June/July 2016 issue in an interactive PDF format.

If an FDA investigation results in a Form 483 then it is important to prove that earlier issues have been resolved upon re-inspection. The following steps using your Corrective and Preventive Action (CAPA) program are crucial to appropriately handling and responding to an FDA Form 483 in helping avoid a Warning Letter.

CRF Health announces reporting enhancements to its TrialManager platform for real-time analysis and management of data collected in clinical trials.


Quintiles announces its Precision Enrollment offering to accelerate site start-up and patient recruitment in oncology clinical trials.


ZRG Partners announce data for the first quarter of 2016 Global Life Sciences Hiring Index.

Pilot study evaluates the feasibility of using wearable devices in clinical data collection, including the training requirements for appropriate use of the mHealth technologies and the impact of the model on data quality and patient engagement.


Cenduit, a joint venture between Quintiles and Thermo Fisher Scientific, announced the release of its upgraded Patient Reminders tool.


Bioclinica releases OnPoint Direct, a Clinical Trial Management System geared toward mid-market biopharma and CROs.


Identifying Key Risk Indicators (KRIs) is an important step in successfully applying risk-based monitoring initiatives to a clinical trial. These factors, within risk management, assist by defining risk areas in order to measure and monitor them centrally throughout the trial.

Identity trust platforms assure clinical investigators that their credentials are legitimate by allowing use of a single identity that can be recognized across multiple entities. These platforms support collaboration by allowing drug development participants to access data, sign and exchange documents.

Regardless of whether the UK does decide to leave the EU or not, the concern over medication will remain for the rest of Europe. The needs of these countries will persist at a human and public health level for more effective medicines and more effective ways of paying for them.

A key function in clinical trials, patient enrollment, has fallen behind during a time where technology has played a vital role in the industry. Adaptive patient recruitment allows for clinical data to be collected and reviewed in real-time as to improve enrollment outcomes as they are taking place.


Bracket releases a new series of mobile applications to support clinical trials.

Autism Spectrum Disorder (ASD) is a common neurodevelopmental disorder which does not have an approved medication to address its core symptoms. The Janssen Autism Knowledge Engine (JAKE) was designed to advance the clinical research process for autism by integrating emerging technologies into traditional clinical trial processes.
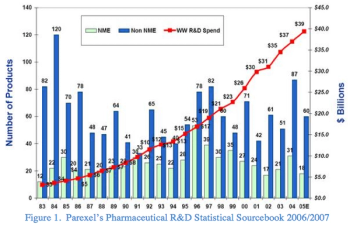
Despite challenges in the pharma sector, we are experiencing a technological shift in the way clinical research data is obtained from patients. New devices have allowed for the collection, reporting and response to data from a patient’s body using sensor technology.
![CyndiVerst[1].png](https://cdn.sanity.io/images/0vv8moc6/act/460bbdd742506542c78e095aa023319ebc3cd470-250x250.png?w=350&fit=crop&auto=format)
Clinical trials of the future may be closer than we think as we strive to deliver smarter, faster and more cost effective treatment to patients. The data exists to make this possible, now we must change our thought process to meet the needs of patients and sponsors.
