
News







CRO giant and leading healthcare information provider merge to create a wide-ranging R&D-to-commerical services company, in a deal some contend could trigger a trend of larger CROs adding complementary non-clinical offerings.



Sponsors are paying more attention to the resources and capabilities of CROs when negotiating research contracts and agreements.

Sponsors and their contractors have faced challenges when ensuring that parties involved in global clinical trials adhere to rules and regulations regarding biomedical research. For an effective collaboration to take place, all parties must meet the expectations and accountabilities detailed in the trial contract.



The Innovative Medicines Initiative, a drug research program run by the European Commission and the EFPIA, is inviting bids to run a pilot program regarding preclinical research and development.

This special report offers an article regarding current FDA thinking and future direction with these assessments. ERT-a cloud platform solutions provider that captures quality efficacy and safety endpoints in centralized Cardiac Safety, Respiratory, Suicide Risk Assessment, and Clinical Outcome Assessments-offers expert view on this regulatory change.

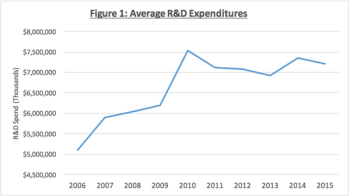
Some in the financial industry have argued that we may be in the midst of another economic recession. The biopharmaceutical industry has shown resiliency during such times and industry trends point to that being the case again.




The Movement Disorders Society has recently published Clinical Diagnostic Criteria for Parkinson’s disease. These new guidelines allow for the diagnosis of clinically established and clinical probable Parkinson’s disease, which will help reduce errors in clinical trials.

With Britain’s potential succession from the EU looming, scientific leaders in Europe are voicing their concerns that losing the UK would be a blow to clinical research.

The European Reference Networks aims to join the efforts of the best specialists in Europe to tackle complex or rare medical conditions. The goal is to create networks covering a specific disease with an emphasis on procedures or techniques related to treatment.
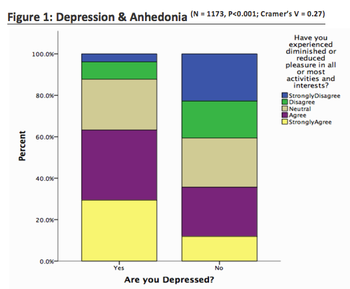
Major Depressive Disorder patients display a reduced ability to feel pleasant experiences known as Anhedonia. Such a feature provides difficulties when treating depression and engaging patients during clinical trials.



Drug safety surveillance, a core focus of clinical trials, can be influenced by subjective judgement, as this analysis of differing expert assessments of adverse drug reactions-and the reasons why-shows.


