
Genocea Biosciences has partnered with Wingspan Technology to implement Wingspan’s compliant electronic TMF system.

Genocea Biosciences has partnered with Wingspan Technology to implement Wingspan’s compliant electronic TMF system.




The Clinical Trials Transformation Initiative (CTTI) released new recommendations for enhancing the efficiency of clinical trial recruitment.


MedNet Solutions and Algorics have announced a partnership to improve risk based monitoring functionality to MedNet’s consumers through the integration of their products.


PAREXEL has opened a North American Coordination Hub and Distribution Center in Pennsylvania.


CDISC and TransCelerate announce new Therapuetic Area Standard for Breast Cancer, which allows for data to be shared among industry professionals.

ERT announces it has successfully integrated the fractional exhaled Nitric Oxide (FeNO) measurement device by Aerocrine, a Circassia subsidiary, into it’s diagnostic platform for spirometry, ECG and home monitoring for centralized clinical trials.

Pfizer has created a clinical trial modeling tool for mitigating study risk during the protocol design and study execution phases. Jonathan Rowe speaks with Moe Alsumidaie on the purpose behind these predictive models.

CRF Health launches Trial Consent, an electronic consent solution that integrates within an eCOA platform.


Oncology remains the therapeutic area with the most drug failures, the lowest numbers of patients enrolled and the highest with the number of drugs in clinical trials. Many trends in oncology clinical trials seek to address these challenges and include the use of biomarkers, immunotherapies, and adaptive designs.

Updated employee announcements, business awards and recognition, and company news.
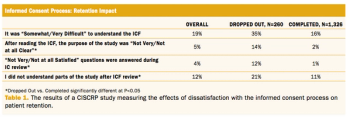
The argument for electronic informed consent as a vital cog in supporting today’s patient-centric push in clinical trials.

The CRO Forum has been asked by TransCelerate to collaborate on reviewing its initiatives. Alan Metz, Forum Chair, and Amy Kissam, Forum Vice-Chair, sit down with Moe Alsumidaie to elaborate on the CRO’s involvement.

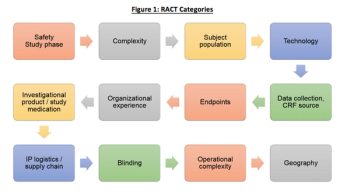
The biopharmaceutical industry is starting to adopt TransCelerate’s Risk Assessment Categorization Tool (RACT) in order to identify risks and plan a comprehensive clinical trial risk mitigation strategy. We recently wrote about the RACT moving to the cloud, and the advantages of using such systems. Some of these advantages include the ability for study teams to evaluate R&D portfolio risks by collecting and analyzing RACT data in aggregate.



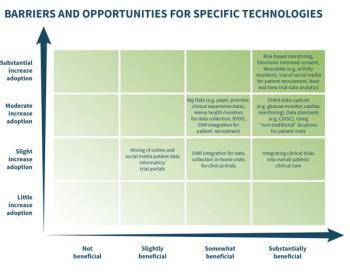
An new survey conducted by the Association of Clinical Research Organizations (ACRO) has identified wearable technology and an increase in social media use for patient recruitment to be two of the more prevalent technology trends in clinical trials.Read the full release here.

The Clinical Data Interchange Standards Consortium (CDISC) announces the availability of a new standard allowing pharmaceutical companies to register their clinical trials from a single files into regulatory databases of the EMA, FDA and WHO.

This special report offers an article regarding current FDA thinking and future direction with these assessments. ERT-a cloud platform solutions provider that captures quality efficacy and safety endpoints in centralized Cardiac Safety, Respiratory, Suicide Risk Assessment, and Clinical Outcome Assessments-offers expert view on this regulatory change.
![Wayne Kubick photo[1].jpg](https://cdn.sanity.io/images/0vv8moc6/act/937ea3115e353ce57e83109baff0c323df1e0723-253x352.jpg?w=350&fit=crop&auto=format)
FHIR (pronounced "fire") is a new, free of cost, platform that has the ability to access and create data though EHR systems. Wayne Kubick writes that using such a platform could truly re-engineer how pharma collects data during clinical trials.
