
News




NAMSA replaced its paper-based system for managing clinical trial documents with Veeva Vault eTMF and has reported that eTMF has reduced the time spent handling files by at least one-third.

PFT Global is the renamed clinical trials division of nSpire Health, which iCardiac acquired in July 2015.



Schulman IRB will serve as the IRB of record for all participating research sites.

The study authors surmised that the lack of validation studies of initial trial results reflected that one goal of open access is not being met. Further, they believe that the limited use indicates a “failure to get the word out “ to researchers.

The impact of the Fourth Industrial Revolution is evident in our everyday lives and healthcare and pharmaceutical industries are no exception and perhaps provide one of the biggest opportunities for a positive impact.

Dr. Christa Wirthumer-Hoche has been elected as the new chair of the Management Board of the European Medicines Agency (EMA).


The dilemma facing clinical operations executives when selecting a CTMS solution to manage clinical trials is to go with an existing approach/solution or explore alternative options.


CRF Health, a provider of eCOA solutions for the life sciences industry, announced its TrialMax® eCOA platform has been selected by a sponsor to capture more than 4.6 million data points via an integrated medical device in a Phase III diabetes program. The program encompasses 3,000 patients across 450 sites in 28 countries using diabetes care devices and patient diaries. Read the full release.

The European Commission has applied to increase the fees payable to EMA by 0.2%, which in line with the 2015 inflation rate.
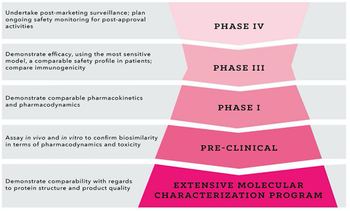
A number of top-selling biological products in key therapeutic areas such as cancer, diabetes, and rheumatoid arthritis have recently lost, or will soon lose, patent protection.

Clinical trials have increased in number and complexity for numerous reasons-global trials, outsourcing, protocol requirements, and multiple regulatory issues. Factor this with the increased need to bring efficiencies and lower prices to the clinical trial process, and the need to stay on top of trials only becomes more crucial. This eBook will include articles on logistics of clinical trials supplies, negotiating with investigative sites, use of e-signatures and more.

If visit information is available in CTMS, Wingspan eTMF automatically creates placeholders for visit documents.


A summary of professional moves in the clinical trials industry, as well as business news and recognition announcements.


MCC's Leadership program is a collaboration of member companies that define industry benchmark measures for Life Science organizations engaged in starting clinical trials.

Clintrax Global provides outsourced services for negotiating clinical trial-related contracts and budgets between biopharmaceutical, CROs and investigator sites.


Ambry Genetics' CEO discusses his company's decision to release the genetic information of its customers for free - a rare move, reportedly, for a commercial testing firm.



Exco InTouch has integrated its ePRO solution with Oracle Health Sciences’ InForm EDC system to provide a data capture solution for both clinical and post-marketing studies.

The CLIA-accredited laboratory will support both research-use-only and clinical diagnostic tests.