
News


Montrium announced the release and availability of a Cloud Qualification Document Pack for Microsoft’s Azure IaaS offering.


INC Research announced a collaboration with CISCRP to develop and implement educational initiatives.

William Looney speaks with DIA's Global Chief Executive to see how the organization is repositioning itself.

Solutions to overcoming the most frequent missteps when collecting valuable spirometry data in respiratory studies.


Medidata has acquired Intelemage, a medical image sharing and workflow management provider.

The agency is concerned about the lack of non-clinical models with good predictive properties in the oncology space.
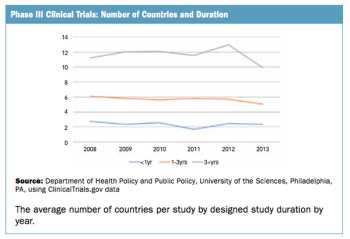
Trend doesn't seem to fall in line with the perception of an increasingly complex trial protocol climate.

Study provides real metrics on the trial-scope affects of protocol changes-shedding fresh light on the importance of adopting new strategies to reduce select amendments.

For programs such as the Precision Medicine Initiative’s Cancer MoonShot to have a chance at success, following a set of key technology mandates will be critical.

Pediatric trials now feature increased modeling and analytics for safer drug dosing and response.
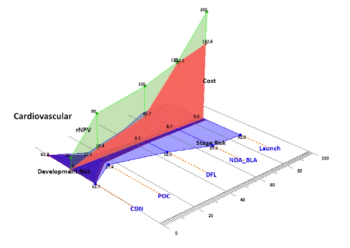
How to integrate evidence-based planning and real-world evidence to boost clinical trial productivity.

How to meet the rigorous safety and efficacy demands critical to evaluating newer targeted cancer therapies.

The smaller biopharmaceutical company perspective on mastering oncology immunotherapy clinical trials.

Click the title above to open the Applied Clinical Trials April/May 2016 issue in an interactive PDF format.


goBalto, Inc., announced its release of goBalto Select, for cloud-based study startup clinical trials.

This article focuses on the 3 essential factors that have a significant impact on the outcome of any inspection or audit. The author applies this primarily to GCP (Good Clinical Practice) inspections, but the principles are universal, being applicable to any government inspection such as the FDA (U.S. Food & Drug Administration) or industry QA (Quality Assurance) audit.
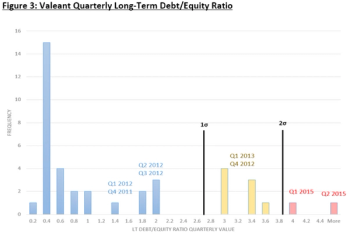
Valeant is a hedge fund disguised as pharmaceutical company, with the moral at the end of it's story, poor ethics leads to collapse.


Pharmacists have new prominence in a new European system, created jointly with drugmakers and wholesalers and parallel traders, that will provide on-line identity-checks on each individual medicine from factory to pharmacy.

Lagging indicators and superficial averages are increasingly replaced by sophisticated performance metrics and predictive analytics harnessing the power of big and small data.


The Mexico office expands Eurotrials reach in Latin America. Eurotrials also has offices in Brazil, Argentina, Chile, Portugal, and Spain, covering over 15 countries in both Latin America and Europe.

The MHRA is seeking input on identifying new uses for an existing drug in another indication, or creating novel combinations and sees it as "an emerging and dynamic field of drug development."


Nimblify, Inc., developer of a patient-centric payment system and a benchmarking tool for sites, among other technologies, will participate as a Global Impact Partner.

Henlius Biotechnology has selected Medidata’s Medidata Clinical Cloud® platform for its Phase III oncology clinical trial in China.