
The Ethical eAdjudication® Platform will join ACRES shared global platform of integrated technologies.

The Ethical eAdjudication® Platform will join ACRES shared global platform of integrated technologies.

The burst of new technology enterprises and innovative service providers that specialize in clinical research is a sign that the clinical research industry is starting to look into new ways to solving problems culminating from an antiquated system.



ActivMed will participate in ACRES ongoing Site Accreditation and Standards Initiative (SASI) to improve performance of global research sites through shared standards, accreditation, training and support.

The discussions will center on ways clinical research can harness gene therapy and new and emerging diagnostic tools.
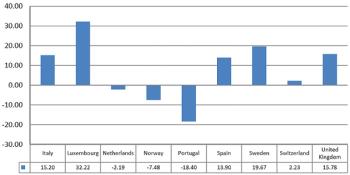
Examining why newly registered trials have fallen in Central and Eastern Europe, relative to other regions.
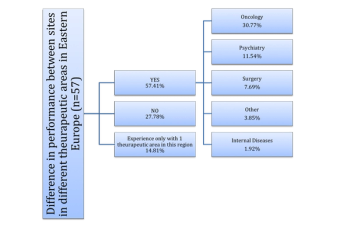
Survey sheds light on the regional-specific risks of managing clinical trial sites in Eastern Europe.

Study uncovers subtle distinctions in attitudes and perceptions among the two groups.


EMA expects a small rise in the pre-authorization activities for human medicines in 2016. Around 546 requests for scientific advice are anticipated, compared to 510 in 2015.

Managed by EMA on behalf of the EU medicines regulatory network, EudraVigilance receives over one million adverse drug reaction reports per year.


Boehringer Ingelheim will integrate Medidata’s study planning, data management, data analytics and RBM capabilities into multiple stages of its drug development process.



Many of the most problematic provisions for health research were eased by amendments that provided exemptions from the overall constraints.


The sponsors will use CRF Health's TrialMax® platform to support data capture for quality of life issues during the post-progression phase.


The December Forum included discussions of global disclosure performance, and highlighted the importance of measuring disclosure performance.





The solution identifies unknown relationships in data, whereby customers can perform dimension-free exploratory analysis on data such as adverse events, labs, concomitant medications, medical history and vital signs.

Bracket SMS allows for reminders to be sent direct to patient mobile phones when they've opted in to the service.
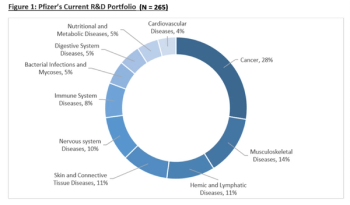
This article evaluates the impact of Allergan’s acquisition on Pfizer’s R&D Portfolio and the long term impact the acquisition will have on Pfizer’s revenue and earnings.

Find out the latest appointments and more in the clinical trials industry.

This overview of clinical supply blinding methods addresses questions about this topic in the context of the current research environment. The recommendations for protecting the blind come at an important time for the industry and may help fill a gap left by the lack of training resources and published material about this subject.