
News



CDER’s Division of Pharmaceutical Analysis (DPA) will be adopting MasterControl’s full QMS to manage documents, training records, audits, CAPAs, change control, and other quality processes.



The findings of analyzed data on clinical trial agreements (CTAs) stored in an online negotiation tool through which sponsors and investigators submit offers and counteroffers on clinical grant costs are shared in this article.




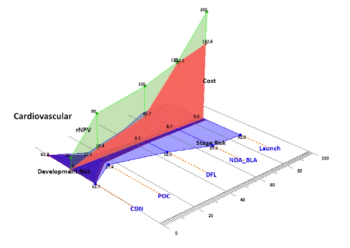
Integrating evidence-based planning and real-world evidence has the potential to reap big gains for development productivity. Achieving this through a "Three-Pillar" approach emphasizing knowledge sharing, evidence needs, and scenario risk-building is proposed.







This scenario seeks to limit animal and clinical studies to only those needed to eliminate residual uncertainty about product performance. Although innovators maintain that clinical trials still are needed to fully examine immunogenicity and other unique attributes of proteins and monoclonal antibodies, biosimilar developers look to extensive product characterization and to pharmacokinetic (PK) or pharmacodynamic (PD) studies to address these concerns.




Applied Clinical Trials is collaborating with the Clinical Endpoints Adjudication Group to conduct an industry survey to evaluate the impact of technology on endpoint adjudication processes.





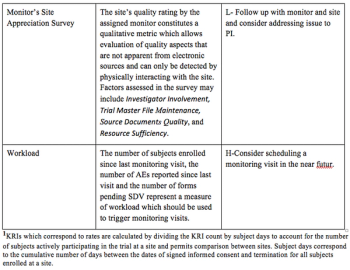
Pilot study evaluates the success of building quality into clinical trials using a plan-do-check-act (PDCA) approach to quality management.

Better access to patient information would enable researchers to answer new questions with existing data, validate findings, and combine the power from individual studies.
