
When paying and reimbursing clinical research volunteers for study participation, what can be done to make the experience more patient centric?

When paying and reimbursing clinical research volunteers for study participation, what can be done to make the experience more patient centric?

Telling signs from U.S., Russia, Latin America and UK indicate that governments are squeezing as much value as possible out of their spending on pills and procedures.

Flex Advantage offers enhanced randomization and clinical supply management capabilities to support adaptive trials.


Private laboratory Biotrial was testing a pain and mood disorder medication for Portuguese pharma company Bial on humans for the first time, in a Phase I trial.
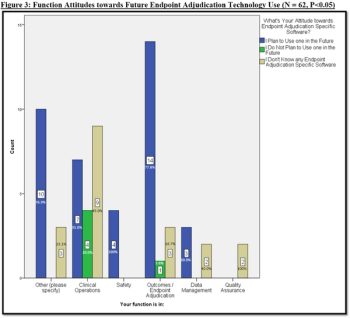
As in other needs in the clinical trials chain, endpoint adjudication is an area that can be greatly improved by technology.

The latest people and business news includes ACRO appointments and awards for CROs and service providers.


Amgen, Celgene, Independence Blue Cross, Bank of America and many more collaborate to deliver immunotherapies more quickly to cancer patients through historic drug development alliance.

BSI, with a long history of publishing multi-industry standards, intends to help develop and implement standards of excellence for clinical research sites.



Move helps biosimulation consulting support for clients in China, as well as support China's expansion of its fast-track drug approval process.

New Phase I clinical in Miami offers gateway location to Latin America.


The Rater Station℠ platform has been used extensively in Central Nervous System clinical trials, and version 4.0 is currently being used in two clinical trials that are actively recruiting.


Early stage development research provider Celerion announced that Vienna, Austria-based Assign Clinical Research has joined the company.

Considerations should be made in regard to social media and clinical trials. Public use and disclosure of an invention before filing for a patent can invalidate the claims and locating evidence to demonstrate public use of an invention can be challenging, particularly if the use is not commercial or publicized.
Pair of surveys provide a rare formal peek into the relationship between sponsors and pediatric clinical research networks. A lack of awareness, incongruent expectations, and insufficient communication have hindered collaboration between the two parties. Developing standardized procedures and better information exchange could help strengthen the connection going forward.


Participant Payments provides clinical research sites the choice to pay participants via reloadable debit card, direct deposit or check.
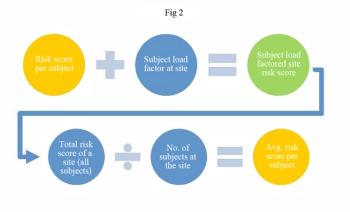
This case study examines using centralized manual data review with statistical approaches to compare value and fit.

For organizations looking to increase transparency in the study startup process, the sheer volume of data and the silos in which they exist can be a daunting hurdle. New generation systems enable teams to capture, analyze, share, and visualize study startup data in one system.

ECT is comprised of two primary services, Protocol+ for the registration of trials and Results Services for the publications of trial results.



The European drug research consortium, IMI2, is running a program entitled Big Data for Better Outcomes Programme to promote "the evolution towards value-based and more outcomes-focused" healthcare, by generating methodologies and data that can inform policy debates.
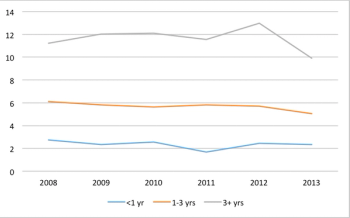
The number of countries used in commercially sponsored Phase III clinical trials has not changed in recent years.