
News

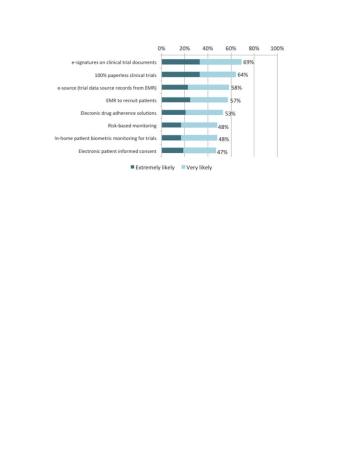
Globally, 80% of respondents reported that all of their staff had access to a desktop computer for clinical data collection, ranging from a low of 66% in India to a high of 89% in Australia.



Despite the benefits of their evidence-based data, RCTs have several disadvantages.

CDER has thrown down the gauntlet to industry on eSource, and now has their work cut out for them on next steps.





Under the partnership, the two companies will work together to deliver OmniComm's electronic data capture (EDC) technology and services integrated with CluePoints' RBM software.


The Patients Alliance for Drug Safety Protections is comprised of 20 public health, patient advocacy, health professional and disease organizations. To underscore the value of REMS to public health, the Alliance unveiled a new online resource.

In this article, you will find a structured summary and critical review of the new addendum, and as well as ideas on how to prepare for these regulatory changes.



Although the devil will be in the detail of the final compromise text of the General Data Protection Regulation (GDPR), we have highlighted below how a few of the proposed changes could potentially impact the pharmaceutical industry.

EMA and FDA regulatory approvals are just the latest events demonstrating the industry’s growing interest in medication adherence measurement in clinical trials, as well as healthcare in general.



Clinical trials today are more commonly assessing quality of life and other PRO measures as part of post-approval studies to present evidence on treatment effectiveness. It is crucial, therefore, that clinical teams have a strong plan for the capture and analysis of PROs data, and the resources required to draw clinically meaningful extrapolations.
Real-world evidence (RWE) research is an increasingly important component of biopharmaceutical product development and commercialization. The growing industry need for broader information on real-world effectiveness and safety-both of which will impact the eventual reimbursement and utilization of new products-is driven by regulators, public and private payers, and prescribers, all of whom seek to better understand the impact of a new product in a real-world setting. The result is that real-world evidence is now included earlier in the research and development phase.
The topic of patient centricity remains very active in the clinical trials industry, as the concept continues to emerge and evolve. The purpose of this Patient Centered Clinical Trials facilitated by our friends at eyeforpharma virtual roundtable is to (a) better understand the definition of patient centricity, (b) uncover challenges associated with industry wide patient centered technology adoption and (c) how patient centricity/engagement technologies will push the biopharmaceutical industry to c




Patient groups “are uniquely positioned” to understand the impact of disease progression, which is central in determining how big and how long clinical trials should be.

Mobile strategies bring near-endless possibilities to engage with patients in clinical trials.