
News









With more than 60 percent of cancers in the United States occurring in people age 65 and older, the evidence base for treating older adults is sparse.

Epic Sciences announced an agreement with LabCorp to provide circulating tumor cell (CTC) technology and support oncology clinical trials in Asia through Covance Drug Development.


Cedars-Sinai announced that its Research for Her online registry that matches women with research studies and clinical trials.


While still early days, key metrics can be collected in three broad areas
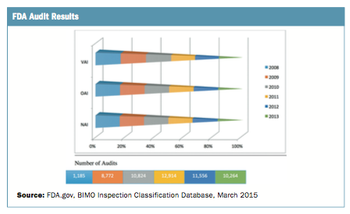
The number of site audits performed has grown significantly.

The four essential steps to ensuring optimal clinical deliverable specifications and process controls.

Whether we are referring to CSRs or to IPD, the personal information of trial participants needs to be de-identified prior to release. This article will describe the available methods for de-identifying clinical trial data, and the relative strengths and weaknesses of each.

CRO/Sponsor: Logical Sourcing: Key Steps Clinical Technology: eSource Record-Keeping Data Sharing: De-identifying Trial Data Also in this issue: R&D Focal Points in Europe Patient Centricity: The Clinical Investment PRO Plusses for Diabetes Research
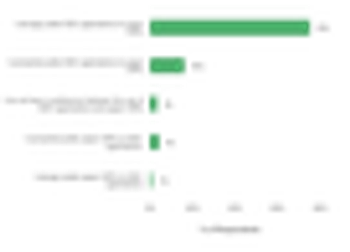
Accuracy in data capture methods has always been and will continue to be a foremost necessity in pharmaceutical clinical trials.

Two Years Successful International Support for Clinical Trials Demonstrates Faster Enrollment, Over 95% Patient Retention Rates.


Over the past three decades, clinical imaging has become an integral part of the drug development process for biopharmaceutical companies.


This new standalone solution is designed to meet the needs of early-stage drug discovery and development researchers.


Contract research organization ICON plc announced a partnership with Mereo BioPharma Group, a specialty biopharmaceutical company.


The collaboration increases the extent of which mHealth tools that can be used to support clinical research.

While the fundamental concerns behind the design of most modern clinical trials are effectively the same, clinical trials that take place during public health emergencies face additional layers of challenges.