
News


Quintiles and Quest Diagnostics announced the launch of Q2 Solutions, their new combined clinical trials laboratory services organization.

There is a lot of buzz about “the cloud.” Formally known as cloud computing, the cloud relies on sharing of Information Technology (IT) resources.


The Rethink Research Competition has opened voting for its People’s Choice Award.



The pilot involved several CDER employees working together with OEA employees to respond to drug related inquiries posted on FDA’s Facebook page.

BioClinica announced the opening of new and expanded offices in London and Munich.


A team of scientists at the University of Vermont has invented a new technique for discovering potentially dangerous drug interactions and unknown side-effects using Twitter hashtags.
A recent survey conducted by Applied Clinical Trials and SCORR Marketing on mHealth in Clinical Trials found the majority of respondents believe mHealth is only beginning its impact on our industry. To the question of challenges to implementing a mHealth strategy, clinical research professionals responded that having the knowledge to execute the skills was the leading challenge.

Acorn Regulatory, an ISO-certified medical device and pharmaceutical consulting firm, has customized programs to help U.S. manufacturers of drug-device combinations in meeting European regulatory approvals.


Medidata announced that its cloud-based technology platform has been adopted by JCR Pharmaceuticals Co., Ltd.

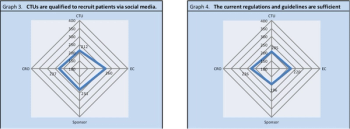
A survey of Belgian clinical research professionals offers insights into the future of social media


The catalog of theranostic markers (TMs), which consists of biomarkers that help to identify patient populations best poised to respond favorably to a specific therapy, continues to expand.

WIRB-Copernicus Group® (WCG), a provider of regulatory and ethical review services and software to support clinical research, announced its 13th annual WCG International Fellows Program in Research Ethics now includes ethics training at New York University (NYU) Langone Medical Center.


Changes in biopharmaceutical R&D strategies and Technological innovations in clinical research are challenging payers on evaluating novel therapeutics and their involvement in the R&D process. While at the 2015 New York BIO annual convention, I had the opportunity to interview Nathan Tinker, Executive Director at the New York Biotechnology Association about how these changes are impacting payers.


Bracket Global, and Clintara announced the two companies have agreed to combine.

The Society for Clinical Research Sites (SCRS) announced a two-year partnership with LabCorp.



The Tufts Center for the Study of Drug Development (CSDD) released a new report highlighting innovative approaches across the R&D continuum that pharmaceutical companies are implementing to drive efficiency and lower cost.