
News


BRANY has successfully continued its Full Accreditation by the Association for the Accreditation of Human Research Protection Programs (AAHRPP).
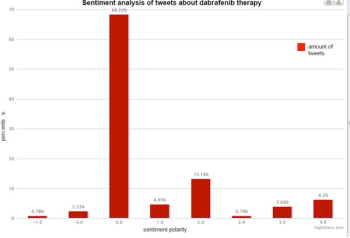
Sentiment analysis can give health care organizations a competitive edge in understanding what customers think about their healthcare experience, to help reduce costs and improve care service and to lead to new clinical research and treatments. In addition, it taps into a new channel of pharmacovigilance input information that can enable Marketing Authorization Holders to keep abreast of opinions on the safety of their products in real time.


WIRB-Copernicus Group (WCG), the world’s largest provider of regulatory and ethical review services and software to support clinical research, announced today that its Western Institutional Review Board® company has been certified to ISO 9001:2008 by BSI.

Over the years, clinical study management has become more fragmented. There are more organizations participating in a study, each bringing their own experiences, interpretations of requirements including how quality is defined. How is it possible that requirements and quality be interpreted so differently?


BBK Worldwide, a clinical trial marketing firm announced it is making the availability of its mobile apps My Clinical Study Buddy®for patients and My Protocol Pal® for investigators free of charge throughout 2015.

Patient focused drug development (PFDD) is moving into the mainstream, promising to alter the conduct of clinical trials and FDA regulatory policies.
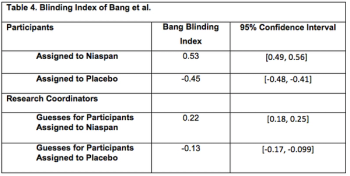
An important criterion for a well-conducted clinical trial is minimization of bias in endpoint assessment.

Biomedical Systems, a premier global provider of centralized diagnostic services, launches its second generation clinical outcome assessment software offering three easy-to-use platforms to collect patient data.

?Lund, Sweden-based Dignitana, a manufacturer of medical cooling devices, engaged CRO Target Health in 2011 toward its approval of DigniCap® System, its product to protect cells and hair for patients in chemotherapy treatment.


Merck Serono, the biopharmaceutical business of Merck, and Illumina, Inc., have agreed to work together to expand the development of a universal next-generation sequencing-based oncology


The Society for Clinical Research Sites (SCRS), a global trade organization dedicated to representing the interests of clinical research sites, announced an expanded relationship with Acurian Inc., a full-service provider of global patient enrollment and retention solutions and a subsidiary of PPD.

MyMeds&Me, a SaaS provider of web-based adverse event and product quality complaint capture solutions for life sciences, announced the deployment of its Reportum® solution across all Pfizer safety call center sites in the United States.


eClinical Solution Enables CTI to Move to a Paperless Global Trial Management Process



In a recent CenterWatch survey, investigative sites rated sponsor companies on more than 36 individual relationship attributes.

The Association of Clinical Research Organizations held its inaugural meeting of the CRO Forum. The Forum was established in October 2014 as the primary vehicle for interaction between the CRO industry and TransCelerate BioPharma.


Dedicated Services to Support Medical Device Manufacturers throughout the Product Lifecycle


SynteractHCR, a full-service, international contract research organization (CRO), has hired Etienne Drouet as vice president, strategic development.

The European Forum for GCP has postponed its multi-stakeholder workshop about “Excluded Older People from Clinical Research,” which was due to be held on 24 March at the Faculty of Medicine/Hospital Clínic de Barcelona, Spain.

Biosimilar approvals in the United States are expected to increase during the next five years, but safety concerns among physicians and the need for greater regulatory clarity concerning therapeutic interchangeability could hinder market uptake.