
I recently visited the Caribbean isles, and on my way at the airport, I noticed prominent CDC disclaimers about an equatorial viral disease: Chikungunya.

I recently visited the Caribbean isles, and on my way at the airport, I noticed prominent CDC disclaimers about an equatorial viral disease: Chikungunya.

Cot? Orphan's new Clinical Research Organization fortifies its foundation as a full-service industry leader in orphan drug regulatory affairs and development.

Published Results Support Recommended Risk-Based Monitoring Methodology

Medidata announced positive results of a data-driven analysis conducted in collaboration with TransCelerate BioPharma

Cot? Orphan announced it will now provide CRO services that will help organizations navigate the clinical trials process

CTI announced that is has opened an office in Geel, Belgium.

New product aims to give CROs and sponsors a competitive advantage

CTI has opened an office in Geel, Belgium

Cost to Develop and Win Marketing Approval for a New Drug Is $2.6 Billion, According to the Tufts Center for the Study of Drug Development

Cost to Develop and Win Marketing Approval for a New Drug Is $2.6 Billion, According to the Tufts Center for the Study of Drug Development
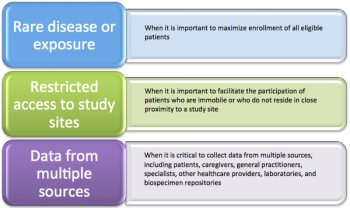
In this article, the authors discuss patient-centered research (also termed "direct-to-patient") that uses the "remote" study approach.

Joint Initiative Evaluates Impact of mHealth and Cloud-Based Technologies on Patient Engagement, Data Quality and Operational Efficiencies in Clinical Trials

Medidata announced the completion of a method development project conducted in partnership with GlaxoSmithKline plc

My recent blog has been overtaken by history, with the departure of Guido Rasi from his post of Executive Director of the European Medicines Agency

Debuting at CNS Summit 2014

Celgene Corporation uses the PHT Corporation SitePad System and StudyWorks to collect patient-driven eData for OTEZLA (apremilast) tablets approved by the FDA in March 2014

Bracket announced the release of Rater Station 3.5

ActiGraph has announced the release of the ActiGraph GT9X Link activity monitor.

Technology Provides Access to Patients Living Far from Study Site

SGS Life Science Services announced that its Biometrics group became a Medidata Rave accredited partner.

SGS Life Science Services announced that its Biometrics group became a Medidata Rave accredited partner.

AMC Health announced the first patient enrolled in a prostate cancer clinical trial deploying televideo

Parexel announced the opening of its new European Coordination Hub and Distribution Center in Berlin-Sch?nefeld.

State-of-the-art facility in Berlin-Sch?nefeld, Germany delivers end-to-end supply chain management and distribution services

NextDocs announced it has formed a partnership with Information Services International-Dentsu, Ltd.

The image acquisition and analysis components of R&D and clinical trials can be quite complex...
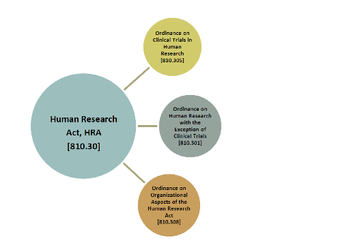
Switzerland is a country small in size, but big in innovation that has always been an attractive location for clinical trials.

Designing protocols to maintain a trial's validity while enabling seriously ill and informed participants to receive both SOC and investigational products will help to improve patient participation in oncology clinical trials.

ICON announced it has been awarded a project by the FDA to develop an industry-standard Patient Reported Outcome