
A new survey conducted on behalf of the NIHR CRN shows that 89% of people would be willing to take part in clinical research if they were diagnosed with a medical condition or disease

A new survey conducted on behalf of the NIHR CRN shows that 89% of people would be willing to take part in clinical research if they were diagnosed with a medical condition or disease

89% of people would be willing to take part in clinical research if they were diagnosed with a medical condition or disease

Patients there respect and trust their doctors and do what he or she tells them to do...like participate in a clinical trial

In the battle against Ebola, incentives from governments and healthcare policymakers have been and will continue to be vital...

BMJ last month posted a research article that shows researchers are more likely to post the results of their trials if they are sent an email reminder.

In the battle against Ebola, incentives from governments and healthcare policymakers have been and will continue to be vital...

Medidata announced the latest release of the Medidata Clinical Cloud

The team behind Clinical Conductor is proud to announce brand new enhancements to the CTMS.

Bio-Optronics has built oncology-specific function into its CTMS, Clinical Conductor

Industry news focusing on the people and organizations who work in the clinical trials profession.

Quintiles Employee Tess Brennan lived with atypical symptoms from a medical condition for more than 20 years

Celerion is pleased to announce membership into the Cardiac Safety Research Consortium.

Applied Clinical Trials wanted to know if Tess had any thoughts or recommendations around clinical trials after being a participant.

Celerion announced its membership into the Cardiac Safety Research Consortium

New Capabilities Optimize Clinical Trial Processes and Enhance Sponsor-Site Relationships, Driving Efficiencies to Streamline Drug Development

New Oncology has entered into a research agreement with Gustave Roussy Cancer Campus in Paris.
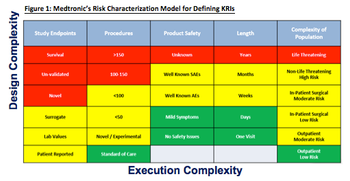
The industry has tried to establish some consistency by standardizing RBM methodologies.

New Oncology announced today that it has entered into a collaboration agreement with Gustave Roussy Cancer Campus, Paris.

Experts have supported the deployment of alternative trial designs to fast-track the evaluation of new Ebola treatments.

Felix A. Khin-Maung-Gyi, executive chairman and founder of Chesapeake Research Review LLC and an ethicist whose field was human research, will be remembered.

ClinicalTrials.gov

Leading health experts today urge the deployment of alternative trial designs to fast-track the evaluation of new Ebola treatments.

PCI has completed a new North American Storage and Distribution facility for Clinical Trial materials.

PCI opens North American Storage and Distribution facility for Investigational Medicinal Products

The organization of healthcare is changing rapidly.

Despite the fact that a growing number of developers and CROs have now had three to five years of experience with these strategic relationships, speed and efficiency improvements and cost savings remain the exception.

To date, Quintiles has been the only CRO with representations at events hosted by the House of Representatives Energy & Commerce Subcommittee on Health's 21st Century Cures initiative.

ERT announced that Provectus Biopharmaceuticals is collaborating with ERT's Clinical Outcome Assessment

To understand just how far mobile and digital technology can truly influence progress in global healthcare, we first need to form the foundation of the discussion with a few rudimentary facts.

During the first six months of 2014, the number of scientific advice and protocol assistance requests for human medicines received by the EMA increased by 16%