
OpenClinica announces that University of Maryland School of Medicine has selected the OpenClinica Enterprise Edition for the Ultra Registry

OpenClinica announces that University of Maryland School of Medicine has selected the OpenClinica Enterprise Edition for the Ultra Registry

The University of Maryland School of Medicine uses OpenClinica's EDC platform to support their multi-center aneurysm trial.

WIRB-Copernicus Group announced that ePharmaSolutions has joined its group of companies.

eDiary System selected to promote data quality and clinical trial efficiencies

What can be gleaned about the direction that health policy will take for the next five years under the new European Commission, scheduled to take office at the beginning of November?

President and Co-founder of Canadian CRO Scimega Research, Denise Deakin, addresses last week's article published in the Toronto Star

Cloud technology is enabling more clinical trial sponsors to meet health authorities' increasing demands for trial master file accessibility and completeness.
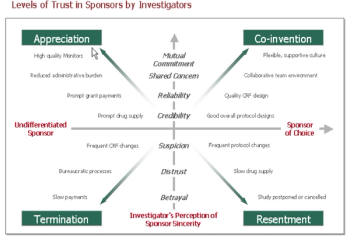
Timely completion of clinical trials is the weak link in the drug development process, and there is no shortage of suggestions for how to improve it.

ClinicalTrials.gov
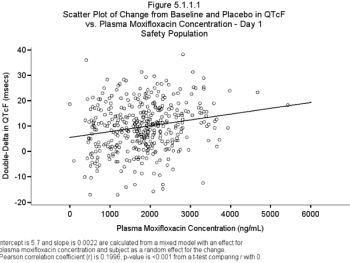
The US FDA, in open meetings, stated the Thorough QT trial "must" be replaced.

Inevitably, the conversation consistently comes back to a simple philosophical debate?should software solutions be configurable or customizable?

WIRB-Copernicus Group announced that its Western Institutional Review Board has been reaccredited

Reaccreditation underscores WIRB-Copernicus Group's commitment to enhancing safeguards for clinical trial participants

Veeva Systems announced updated results of a large TMF survey

Cloud technology enables clinical trial sponsors to meet health authorities' increasing rigor for trial master file accessibility and completeness

A Polish legal expert and the German/Canadian President of the European Federation of Pharmaceutical Industries and Associations will be the co-chairs of the next Drug Information Association EuroMeeting.

The current regulatory system for medicines in Europe could be used in a more efficient and effective manner, according to a report from Escher.

A new report from Escher, the independent TI Pharma platform for regulatory innovation, shows that the current regulatory system for medicines in Europe can be used in a more efficient and effective manner.

AAHRPP announced that it has accredited three more organizations.

The Association for the Accreditation of Human Research Protection Programs today announced that it has accredited three more organizations

There are various estimates in the public domain that applying big data strategies could potentially generate up to $100 billion in value annually across the US healthcare systems.

Members boast 100,000 employees, conduct clinical trials in 142 countries

Members of the Association of Clinical Research Organizations have more than doubled in size in the past 10 years

Researchers will share and analyze data to improve lives of patients living with chronic kidney disease and end stage renal disease

ACRES announced Phase 3 of its Site Accreditation and Standards Initiative

ACRES launches 3rd phase of collaborative effort

Covance and Frenova Renal Research announced a new research collaboration

The rush is on to develop new therapies and preventives to combat the lethal Ebola outbreak.

Cluepoints announced that its CSM platform has been used to assess the quality and integrity of data in a Phase III study of gastric cancer.

Regulatory Intelligence 101 serves as an essential guide to the basics of conducting regulatory intelligence.