
Patients 65 years of age and over are underrepresented in clinical trials, even though in many therapeutic categories they are the primary drug consumers.

Patients 65 years of age and over are underrepresented in clinical trials, even though in many therapeutic categories they are the primary drug consumers.

New Integrated Platform Streamlines, Guides Genomic Analysis for Clinical Trials

Omicia, Inc. announced that its clinical next-generation sequencing interpretation software will be used by LabCorp.

We compiled a list of how sponsors offer access to patient level clinical data requests from researchers.
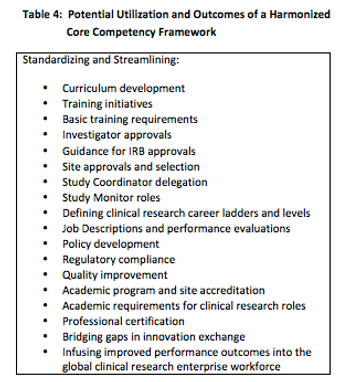
Medicines development and clinical research are among the most heavily regulated activities on a global basis.

Eli Lilly and Company announced it will begin sharing its clinical trial data with scientific researchers through www.clinicalstudydatarequest.com.

Will conduct the first comprehensive in-depth analysis of outsourced clinical research quality.

While at the 2014 New York BIO Conference, I had the opportunity to interview Nathan Tinker, Executive Director at the New York Biotechnology Association, about challenges in early phase development.

Thomson Reuters Cortellis Competitive Intelligence


Clinical trial optimization through adaptation in several key areas was a prevailing message at the recent Partnerships Conference.

Congressional leaders have launched a major initiative to revise laws and regulatory policies to speed new cures to patients.


How iterative analysis of subject recruitment data can optimize outcomes during ongoing enrollment.

CRO/Sponsor: High Performing Study Startups Sites: RBM Challenges, Opportunities Trial Design: Three-pronged Monitoring Approach Study Recruitment: Enhancing Trial Enrollment Also in this issue: New EU Drug Legislation The Patient Experience Rebranding RBM

Drug development in oncology has some of the lowest rates of success and clinical trials in oncology are difficult because of recruitment, challenging protocols and multiple technologies used to evaluate drugs in trials. This eBook contains information on these challenges including Phase I trials, to improving recruitment and trends in oncology clinical trials.

This commemorative e-book includes special articles, as well as DIA session highlights and news that pharmaceutical and clinical trial professionals will find important and informative.

NAPF is launching its Project Innovation, a national movement to accelerate the pace of cancer medical discovery.

Project Innovation Launched to Drive Action

The WIRB-Copernicus Group announced it has formed a new cancer-focused institutional review board, WCG Oncology.

inVentiv Health Clinical and Advaxis, Inc. announced they have entered into a global master services agreement...

A European bid to impose additional limits on research involving human embryos has been defeated.

The WIRB-Copernicus Group announced today the formation of a new cancer-focused institutional review board, WCG Oncology

Redacted policy on sharing drug trial data in Europe.

If the EMA also stops releasing data on old trials, everybody will be kept even more in the dark, according to an editorial posted by bmj.com on May 28.

Celerion and the Korean Drug Development Fund announced a formal collaboration

Jean Burns shared experiences with me that offer important insights for government and industry funded clinical research sponsors to consider as they look to improve their partnership with study volunteers.

Celerion and the Korean Drug Development Fund announced a formal collaboration

INC Research announced a two-year partnership with the Society for Clinical Research Sites.

Effective clinical trial management depends on accurate and unbiased performance measurement.