
With all of the activity in CRO M&As the past three years, it?s slightly amazing that it continues...

With all of the activity in CRO M&As the past three years, it?s slightly amazing that it continues...

Greenphire announced it has released new visual analytics functionality for its eClinicalGPS technology

Greenphire announced the release of new visual analytics functionality for its eClinicalGPS technology.

In November 2013, India's Drug Controller General required that all clinical trials must record on video a participant's informed consent.

Logi Info Embedded Into Core Applications to Help Advance Clinical Trial Research
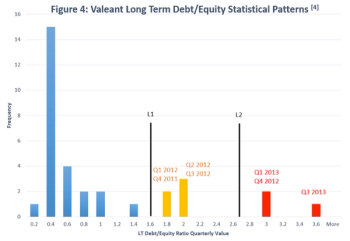
Douglas Tsao, Vice President at Barclays, spoke on the outcomes of Merges & Acquisitions, and its impact on company stock prices.

MCC has just published an executive summary of its Risk Based Monitoring Usage Survey

Theorem Clinical Research recently expanded its Medidata partnership by obtaining accreditation for Medidata Patient Cloud.

Theorem Clinical Research has expanded its Medidata partnership
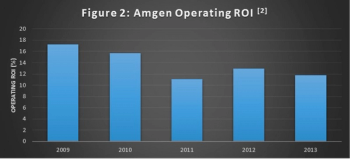
While at the 2014 Partnerships in Clinical Trials Conference, Adrian Otte, VP of Global Development Operations at Amgen, spoke about how Amgen's Risk-Based Monitoring and FSP models impacted business outcomes.
An industry and academic data-sharing project went live on Tuesday, nearly a year after the platform was expected to launch.

ePharmaSolutions, a provider of eClinical solutions, announced positive results of initial pilot studies for its patient matching and triaging solution, ReferralPlus.

A new report from research and consulting firm GlobalData forecasts the neuropathic pain market value to increase from $2.58 billion in 2012 to $3.53 billion by 2022

A management report from Tufts CSDD, based on an executive roundtable, found that drug companies and their development partners are incorporating business planning earlier in clinical development

Almac's diagnostics business unit announced it has received a Clinical Laboratory Permit from the New York State Department of Health

Lisa Henderson recaps Roni Zeiger's keynote from the Partnerships in Clinical Trials conference earlier in April.
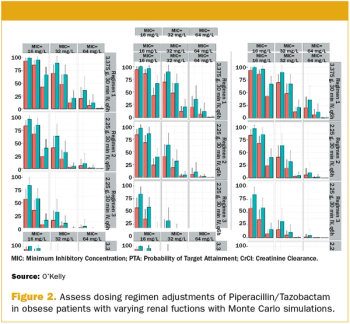
Computer-based modeling and simulation has advanced numerous industries, from aeronautics and engineering to meteorology and finance.

Almost all of us have said over the years, "With the things I?ve seen, I should write a book."

Global neuropathic pain treatment market value to increase from $2.58 billion in 2012 to $3.53 billion by 2022

Drug companies and their development partners who are seeking to increase clinical success rates of new drug candidates are developing tools

Certara announced its acquisition of specialty contract research organization Synchrogenix Information Strategies Inc

Almac's diagnostics business unit today announced that it has received a Clinical Laboratory Permit from the New York State Department of Health

Purchase adds global regulatory writing and submissionservices to Certara?s drug development consulting expertise

inVentiv Health Clinical has been selected for an osteoarthritis Investigational New Drug Application by IntelliCell BioSciences, Inc

BioPharm Systems, a global IT consulting and managed services provider in the life sciences industry, has been acquired by Perficient.

Perficient announced it has acquired BioPharm Systems, Inc.

The European Medicines Agency is determined to minimize opposition to its next moves on releasing clinical trial data.

Firecrest platform offers unique capabilities for improving the quality, consistency, and compliance of ICH/GCP

ICON announced that TransCelerate has certified its Firecrest GCP training as mutually acceptable for its members.

RAPS announced a new chapter in Taiwan, which is its first in Asia.