
The Biotechnology Industry Organization today reaffirmed and broadened its long-standing commitment to improving human health...

The Biotechnology Industry Organization today reaffirmed and broadened its long-standing commitment to improving human health...

NEOVACS announced its financial results for the year to 31 December 2013 as approved by the Board of Directors on March 26th 2014.

Novel scientific collaboration to use genomics and big data to drive drug discovery

MedNet will once again be participating in the Partnerships in Clinical Trials conference.

A public-private research initiative between GSK, the European Bioinformatics Institute and the Wellcome Trust Sanger Institute aims to harness the power of "big data" and genome sequencing to improve the success rate for discovering new medicines.

As payers demand more evidence documenting medical product value, biopharma companies are responding by moving sooner to decide key clinical outcomes to measure.

The global orphan drugs market presents many opportunities for new drug development...

PCORI has appointed 10 members to its new Advisory Panel on Clinical Trials

PCORI has launched its Matchmaking App Challenge

Panel Will Provide Expertise on Trial Selection, Design and Implementation

Frost & Sullivan: Breakthrough therapies for rare diseases command premium pricing, particularly if no alternatives exist

New investments in facilities and scientific leadership reflect Covance?s commitment to biologics development

Oracle announced its Clinical Monitoring Cloud

Covance announced it is expanding its large molecule bioanalytical space


PHT Corporation is offering an app based on the FDA's Clinical Outcome Assessment Qualification Program

The shift to personalized medicine has been hindered by uncertainty over the value, accuracy, and clinical utility of companion diagnostic tests.

Adaptive licensing has leapt from the pages of learned journals into the real world of European regulation.

The last decade has seen the complexity, size, and costs of clinical trials increase, which has made the task of guaranteeing data quality progressively difficult.

The FDA Roadmap enables researchers involved in patient-centric trials to navigate FDA clinical outcome assessment qualification program

Mosio has launched an eBook "Clinical Trials Patient Recruiting and Retention: Tips for Success."
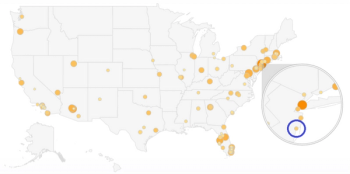
This article will describe my experiences in creating robust strategies for optimizing site selection and offer a few recommendations.

Medis Research Group has been joined by ONKODATAMED GmbH in a bid to consolidate the group's position as an oncology trial specialist...

Also accredited: first organization in the Middle East, second in Taiwan, and St. Jude Children's Research Hospital

Industry news focusing on the people and organizations who work in the clinical trials profession.

Rescop announced today the opening of their offices in the UK and Australia.

Improving timely access for patients to new medicines: pilot explores adaptive licensing approach with real medicines in development

EMA has launched an adaptive licensing pilot project designed to improve access to new medicines...

AAHRPP announced it has accredited four more organizations