
Rescop )Regulatory System Compliance Partners) has opened new offices in the UK and Australia.

Rescop )Regulatory System Compliance Partners) has opened new offices in the UK and Australia.

PAREXEL has expanded its global Clinical Logistics Services capabilities for global clinical trial supply

Increases global capacity and adds new secondary packaging and labeling services to more efficiently deliver clinical trial supplies

MMG announced an exclusive strategic partnership with online healthcare communications company MedRespond

MMG announced an exclusive strategic partnership with online healthcare communications company MedRespond to introduce Custom Conversations
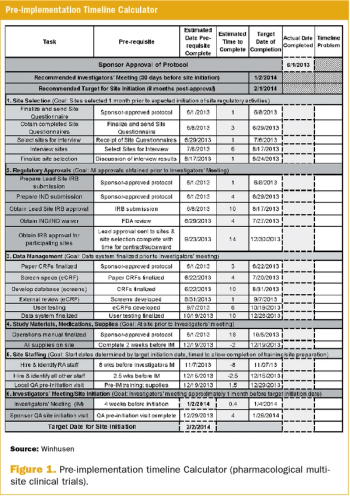
Increasing the speed with which promising pharmacological treatments move from pre-clinical research to patient-care utilization has received increasing focus in recent years.

Full service CRO WCCT Global is opening a new Asian Bridging clinical research site.

ERT has received authorization to provide electronic versions of the St. George?s Respiratory Questionnaires on SITEpro Tablet

Clinical Research Management, Inc. announced today it was awarded a contract by the Biomedical Advanced Research and Development Authority.

New Asian Bridging Clinical Research Unit has been opened by Contract Research Organization WCCT Global

ERT today announced it has received authorization to provide electronic versions of the St. George's Respiratory Questionnaires on SITEpro Tablet

Clinical Research Management has been selected by the HHS' Biomedical Advanced Research and Development Authority

Expertise to aid development of novel therapies to respond to terrorist attacks, pandemics

On March 13, the European Medicines Agency published updated information about current tenders...

PPD has been selected by the HHS' Biomedical Advanced Research and Development Authority in its Medical Countermeasure Clinical Studies Network.

ACRP is organizing an online teaching session about regulatory inspections, to be held on May 14, 2014.

C3i announced that it has been selected by CFS Clinical to provide global 24x365 multilingual technology support services for their Site Activation and Investigator Payment Services.

Agreement Focuses on Development of Clinical Specimen Repositories, Laboratory Services, and Research.

Brought to you by Applied Clinical Trials, The Clinical Data Disclosure and Transparency Resource Community is the go-to web destination for comprehensive data disclosure and transparency content.

CFS Clinical has selected C3i, a service desk support and business process outsourcing services provider.

MRIGlobal and the University of Maryland School of Medicine announced a two- year agreement...

In an effort to reduce costs and improve quality of research, clinical trials are increasingly being offshored.

Biopharmaceutical companies are treading lightly in the use of websites, chat rooms, and interactive online communications to support clinical research programs.

An interview with Dr. Greg Koski about the Alliance for Clinical Research Excellence and Safety (ACRES).

The OnCore system will also support clinical research operations at its new world-class facility set to open in 2016.

DSG's suite of eClinical solutions chosen for Phase III study of new treatments for acute uncomplicated influenza.
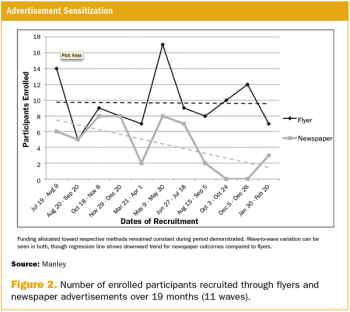
Clinical trials often fail to reach desired goals due to poor recruitment outcomes, including low participant turnout, high recruitment cost, or poor representation of minorities.

Choice Pharma provides Clinipace a significant Pan-Asia footprint

Comprehensive support for trials of any size or complexity whether they originate in Asia or include Asian sites

Choice Pharma provides Clinipace a significant Pan-Asia footprint