
Industry news focusing on the people and organizations who work in the clinical trials profession.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Pharm Exec spoke with leaders in a resurgent patient movement about compassionate use access to new drugs, clinical trial participation policy, and the individual unmet needs of patients suffering from dementia and cancer.

Product helps to determine whether a drug candidate prolongs cardiac repolarization, a surrogate for potentially-fatal arrhythmias

Product helps to determine whether a drug candidate prolongs cardiac repolarization, a surrogate for potentially-fatal arrhythmias

ZRG Partners, Inc released its 4th Quarter Life Science Hiring Index.

ZRG Partners, Inc. announces the results of its fourth quarter Global Life Sciences Hiring Index

ROI from clinical research can suffer when too many research organizations seek to develop treatments for similar conditions and indications

New solution leverages linked data technologies to power the discovery of new scientific and strategic insights

ACRP GCP Courses Recognized for Good Clinical Practice by TransCelerate Biopharma

Rare Disease Management announced its rare disease clinical trial feasibility tool

New solution leverages linked data technologies to power the discovery of new scientific and strategic insights


First-of-its-Kind Facility to Begin Operations in Early Spring

The DIA has announced that its early registration fee reduction for next month's EuroMeeting will end on Tuesday February 11.

PCORI is launching new programs that are expected to encourage more analysis of the effectiveness of specific drugs and medical products...

The NIH, along with 10 biopharma companies and eight non-profit organizations, have formed the Accelerating Medicines Partnership

New Full-Service Projects Drive 114% Increase in Bookings Over FY2012

No less than 11 out of 81 medicines recommended for marketing authorization in 2013... were intended for the treatment of rare diseases

Goal is to develop new treatments earlier, beginning with Alzheimer's, type 2 diabetes, and autoimmune disorders.

Building an accurate and consistent billing compliance process is a complex task.

Clinipace Worldwide has announced that 2013 was its strongest year to date.
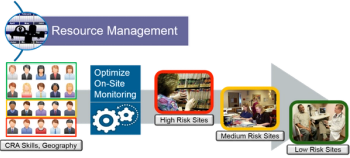
How does one determine and define the "risk" in a risk-based monitoring approach for clinical trials?

CTI Clinical Trial and Consulting Services announces the acquisition of Community Research

CTI Clinical Trial and Consulting Services has acquired a multispeciality research site with three locations throughout Greater Cincinnati, Ohio.

The FDA plans to issue numerous draft and final guidances in the coming months...

An insight into how EU membership impacts on medicines and pharmaceuticals has appeared from a British politician

There are a variety of CTMS applications on the marketplace today, and organizations sometimes have a hard time selecting the system that will work the best for them.

Oracle announced it will integrate Nuance Communications cloud-based medical speech recognition

The Washington health policy world is headed for a major shake-up next year, as more long-time leading legislators opt for retirement.