
Gastrointestinal therapeutics market to decline from $6.8 billion in 2012 to $6.6 billion by 2019

Gastrointestinal therapeutics market to decline from $6.8 billion in 2012 to $6.6 billion by 2019

Further details about the 17th International Conference on Pharmaceutical Medicine (ICPM 2014) have been unveiled.

The European Forum for GCP (EFGCP) has published its final report, ?Indemnity Schemes for Clinical Trials: A Societal Obligation??

attend the 17th International Conference on Pharmaceutical Medicine (ICPM 2014). It is an international forum for pharmaceutical physicians, pharmaceutical medicine professionals and clinical research experts world-wide who are involved in the discovery, development, evaluation, registration, monitoring and marketing of medicines.

The U.S. Food and Drug Administration opened the door to a new era of clinical trial management last summer when it released guidance for industry on clinical trial oversight and called for expanded use of risk-based monitoring.

51% of the overall gastrointestinal therapeutic pipeline is in Phase II to Phase III development, of which 19 molecules are indicated for the treatment of Irritable Bowel Syndrome, 48 for ulcerative colitis, and 41 for Chron?s Disease.

New report shows life sciences sector using benefits to win talent war

Salaries in the U.K. life sciences sector are static, but the range of non-monetary benefits being offered to staff is improving fast, according to a new salary survey.

With the upcoming release of Wingspan eTMF 2.0, Wingspan Technology is holding an exclusive preview webinar

Wingspan Technology will debut its eTMF 2.0 via webcast January 22.

The Association of Clinical Research Professionals (ACRP) has learned that TransCelerate Biopharma, Inc., now recognizes certification through the Certified Clinical Research Coordinator (CCRC?) and Certified Physician Investigator (CPI?) programs of ACRP's affiliate, the Academy of Clinical Research Professionals (the Academy), as evidence of Good Clinical Practice (GCP) training.

Clinical research professionals who have a current CPI or CCRC designation will be able to use that as evidence that retaking GCP training is not necessary if they are working on a trial for one of the TransCelerate companies.

A recent report from the Business Monitor outlines India's strong commercial opportunities for pharmaceutical companies...

Global IT spending will increase at a cumulative annual growth rate (CAGR) of 3.6% to reach $40.8bn by 2017, predicts Ovum

ComprehensiveOffering Provides Flexible Access to Key Quality Processes

MediGuard App Wins Web Health Award

Business Monitor has just released its latest findings on India's pharmaceuticals and healthcare sector in its newly-published India Pharmaceuticals & Healthcare Report.

Sparta Systems announced that is offering mobile versions of selected TrackWise product suites.

MEDIAN Technologies announced that it has signed a contract with a San Francisco-area based biotech company

MEDIAN Technologies will provide imaging services for a phase II study on lung cancer
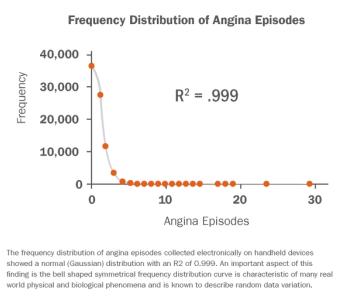
Eight million people in the US alone suffer from chronic stable angina, the chest pain and tightness associated with myocardial ischemia caused by coronary artery disease

FDA Purchases Novel ADDPLAN DF Software for Improved Evaluation of Phase II Dose-Finding Trials

Industry Leaders Share Expertise through Complimentary White Paper on ePRO Regulatory Inspections

IMS Health has filed a registration statement with the US Securities and Exchange Commission

IMS Health today announced that it has publicly filed a registration statement with the US Securities and Exchange Commission

Theorem Clinical Research has announced the formation of a strategic alliance with BioTelemetry, Inc.

With 2013 having come to a close and gearing up for a what's sure to be a fantastic 2014, it seems an appropriate time to reflect back on some of the most popular content of the year.

Significant Genotypic and Phenotypic Analytic Capabilities Added in New Release

CROS NT Ltd announced the acquisition of MDSL International

As part of the CROS NT Group global growth strategy, CROS NT Ltd today announced the acquisition of MDSL International